Analysis of human C4A and C4B binding to an immune complex in serum
- PMID: 10403910
- PMCID: PMC1905463
- DOI: 10.1046/j.1365-2249.1999.00940.x
Analysis of human C4A and C4B binding to an immune complex in serum
Abstract
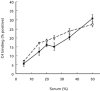
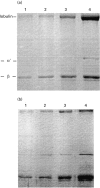
Previous studies using isolated complement proteins have shown that more C4A than C4B binds to certain types of immune complexes. However, the in vivo binding of the C4 isoforms to an immune complex has not been investigated in detail and may differ from events when measured with the isolated proteins. We report here the binding of C4A and C4B to an immune complex of bovine serum albumin (BSA) anti-BSA as it occurs in serum. We found that when using the isolated C4 proteins more C4A than C4B bound to the complex, but in serum similar amounts of C4A and C4B were found to bind. Furthermore, these results were not explainable by a difference in activity between isoforms. In an attempt to explain these results a number of unexpected observations were noted. First C4A, but not C4B, bound specifically to a yet unidentified 38-kD serum protein. Second, when both covalent and non-covalent binding was assessed, we found that as serum concentration increased there followed a concomitant decrease in covalent binding and C4B was more affected than C4A. The potential biological significance of these findings is discussed.
Figures






Similar articles
-
Differences between C4A and C4B in the handling of immune complexes: the enhancement of CR1 binding is more important than the inhibition of immunoprecipitation.Clin Exp Immunol. 1990 Feb;79(2):158-63. doi: 10.1111/j.1365-2249.1990.tb05172.x. Clin Exp Immunol. 1990. PMID: 2138067 Free PMC article.
-
Glomerular deposition of the complement C4 isotypes C4A and C4B in glomeruonephritis.Nephrol Dial Transplant. 1996 Jun;11(6):1024-8. Nephrol Dial Transplant. 1996. PMID: 8671963
-
Covalent binding properties of the human complement protein C4 and hydrolysis rate of the internal thioester upon activation.Protein Sci. 1993 May;2(5):706-16. doi: 10.1002/pro.5560020502. Protein Sci. 1993. PMID: 8495193 Free PMC article.
-
Two isotypes of human C4, C4A and C4B have different structure and function.Complement Inflamm. 1989;6(1):19-26. doi: 10.1159/000463068. Complement Inflamm. 1989. PMID: 2650988 Review.
-
Inherited deficiency of the fourth component of human complement.Immunodefic Rev. 1988;1(1):3-22. Immunodefic Rev. 1988. PMID: 3078708 Review.
Cited by
-
A CR1 polymorphism associated with constitutive erythrocyte CR1 levels affects binding to C4b but not C3b.Immunology. 2003 Apr;108(4):531-8. doi: 10.1046/j.1365-2567.2003.01579.x. Immunology. 2003. PMID: 12667215 Free PMC article.
-
Early Components of the Complement Classical Activation Pathway in Human Systemic Autoimmune Diseases.Front Immunol. 2016 Feb 15;7:36. doi: 10.3389/fimmu.2016.00036. eCollection 2016. Front Immunol. 2016. PMID: 26913032 Free PMC article. Review.
References
-
- Sim E, Dodds AW. The fourth component of human complement—towards understanding an enigma of variations. In: Whaley K, editor. Complement in health and disease. Lancaster: MTP Press Ltd; 1987. p. 99.
-
- Campbell RD, Law SKA, Reid KBM, Sim RB. Structure, organization and regulation of the complement genes. Ann Rev Immunol. 1988;6:161–95. - PubMed
-
- Belt KT, Carroll MC, Porter RR. The structural basis of the multiple forms of human complement component C4. Cell. 1984;36:907–14. - PubMed
-
- Belt KT, Yu CY, Carroll MC, Porter RR. Polymorphism of human complement component C4. Immunogentics. 1985;21:173–80. - PubMed
-
- Law SKA, Lichtenberg NA, Holcombe FH, Levine RP. Interaction between the labile binding sites of the fourth (C4) and fifth (C5) human complement proteins and erythrocyte cell membranes. J Immunol. 1980;125:634–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

