Comparison of Ehrlichia chaffeensis recombinant proteins for serologic diagnosis of human monocytotropic ehrlichiosis
- PMID: 10405403
- PMCID: PMC85285
- DOI: 10.1128/JCM.37.8.2568-2575.1999
Comparison of Ehrlichia chaffeensis recombinant proteins for serologic diagnosis of human monocytotropic ehrlichiosis
Abstract
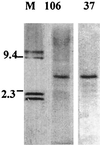
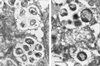
Diagnosis of human monocytotropic ehrlichiosis (HME) generally depends on serology that detects the antibody response to immunodominant proteins of Ehrlichia chaffeensis. Protein immunoblotting was used to evaluate the reaction of the antibodies in patients' sera with the recombinant E. chaffeensis 120- and 28-kDa proteins as well as the 106- and the 37-kDa proteins. The cloning of the genes encoding the latter two proteins is described in this report. Immunoelectron microscopy demonstrated that the 106-kDa protein is located at the surfaces of ehrlichiae and on the intramorular fibrillar structures associated with E. chaffeensis. The 37-kDa protein is homologous to the iron-binding protein of gram-negative bacteria. Forty-two serum samples from patients who were suspected to have HME were tested by immunofluorescence (IFA) using E. chaffeensis antigen and by protein immunoblotting using recombinant E. chaffeensis proteins expressed in Escherichia coli. Thirty-two serum samples contained IFA antibodies at a titer of 1:64 or greater. The correlation of IFA and recombinant protein immunoblotting was 100% for the 120-kDa protein, 41% for the 28-kDa protein, 9.4% for the 106-kDa protein, and 0% for the 37-kDa protein. None of the recombinant antigens yielded false-positive results. All the sera reactive with the recombinant 28- or the 106-kDa proteins also reacted with the recombinant 120-kDa protein.
Figures



Similar articles
-
Western and dot blotting analyses of Ehrlichia chaffeensis indirect fluorescent-antibody assay-positive and -negative human sera by using native and recombinant E. chaffeensis and E. canis antigens.J Clin Microbiol. 1999 Dec;37(12):3888-95. doi: 10.1128/JCM.37.12.3888-3895.1999. J Clin Microbiol. 1999. PMID: 10565902 Free PMC article.
-
Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family.Infect Immun. 1998 Jan;66(1):132-9. doi: 10.1128/IAI.66.1.132-139.1998. Infect Immun. 1998. PMID: 9423849 Free PMC article.
-
Cloning and characterization of multigenes encoding the immunodominant 30-kilodalton major outer membrane proteins of Ehrlichia canis and application of the recombinant protein for serodiagnosis.J Clin Microbiol. 1998 Sep;36(9):2671-80. doi: 10.1128/JCM.36.9.2671-2680.1998. J Clin Microbiol. 1998. PMID: 9705412 Free PMC article.
-
Tick-borne ehrlichiosis infection in human beings.J Vector Borne Dis. 2008 Dec;45(4):273-80. J Vector Borne Dis. 2008. PMID: 19248653 Review.
-
Ehrlichiae and ehrlichial diseases in china.Ann N Y Acad Sci. 2003 Jun;990:45-53. doi: 10.1111/j.1749-6632.2003.tb07335.x. Ann N Y Acad Sci. 2003. PMID: 12860598 Review.
Cited by
-
Survival strategy of obligately intracellular Ehrlichia chaffeensis: novel modulation of immune response and host cell cycles.Infect Immun. 2004 Jan;72(1):498-507. doi: 10.1128/IAI.72.1.498-507.2004. Infect Immun. 2004. PMID: 14688131 Free PMC article.
-
Ehrlichia chaffeensis: a prototypical emerging pathogen.Clin Microbiol Rev. 2003 Jan;16(1):37-64. doi: 10.1128/CMR.16.1.37-64.2003. Clin Microbiol Rev. 2003. PMID: 12525424 Free PMC article. Review.
-
Molecular characterization of antibody epitopes of Ehrlichia chaffeensis ankyrin protein 200 and tandem repeat protein 47 and evaluation of synthetic immunodeterminants for serodiagnosis of human monocytotropic ehrlichiosis.Clin Vaccine Immunol. 2010 Jan;17(1):87-97. doi: 10.1128/CVI.00331-09. Epub 2009 Dec 2. Clin Vaccine Immunol. 2010. PMID: 19955322 Free PMC article.
-
Identification of a p28 gene in Ehrlichia ewingii: evaluation of gene for use as a target for a species-specific PCR diagnostic assay.J Clin Microbiol. 2001 Nov;39(11):3871-6. doi: 10.1128/JCM.39.11.3871-3876.2001. J Clin Microbiol. 2001. PMID: 11682500 Free PMC article.
-
Expression of a gene encoding the major antigenic protein 2 homolog of ehrlichia chaffeensis and potential application for serodiagnosis.J Clin Microbiol. 2000 Oct;38(10):3705-9. doi: 10.1128/JCM.38.10.3705-3709.2000. J Clin Microbiol. 2000. PMID: 11015387 Free PMC article.
References
-
- Adhikari P, Kirby S D, Nowalk A J, Veraldi K L, Schryvers A B, Mietzner T A. Biochemical characterization of a Haemophilus influenzae periplasmic iron transport operon. J Biol Chem. 1995;270:25142–25149. - PubMed
-
- Anderson B E, Sims K G, Olson J G, Childs J E, Piesman J F, Happ C M, Maupin G O, Johnson B J B. Amblyomma americanum: a potential vector of human ehrlichiosis. Am J Trop Med Hyg. 1993;49:239–244. - PubMed
-
- Anderson B E, Greene C E, Jones D C, Dawson J E. Ehrlichia ewingii sp. nov., the etiologic agent of canine granulocytic ehrlichiosis. Int J Syst Bacteriol. 1992;42:299–302. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

