alpha-Synuclein shares physical and functional homology with 14-3-3 proteins
- PMID: 10407019
- PMCID: PMC6783081
- DOI: 10.1523/JNEUROSCI.19-14-05782.1999
alpha-Synuclein shares physical and functional homology with 14-3-3 proteins
Abstract
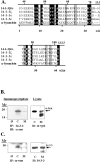
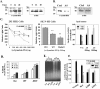
alpha-Synuclein has been implicated in the pathophysiology of many neurodegenerative diseases, including Parkinson's disease (PD) and Alzheimer's disease. Mutations in alpha-synuclein cause some cases of familial PD (Polymeropoulos et al., 1997; Kruger et al., 1998). In addition, many neurodegenerative diseases show accumulation of alpha-synuclein in dystrophic neurites and in Lewy bodies (Spillantini et al., 1998). Here, we show that alpha-synuclein shares physical and functional homology with 14-3-3 proteins, which are a family of ubiquitous cytoplasmic chaperones. Regions of alpha-synuclein and 14-3-3 proteins share over 40% homology. In addition, alpha-synuclein binds to 14-3-3 proteins, as well as some proteins known to associate with 14-3-3, including protein kinase C, BAD, and extracellular regulated kinase, but not Raf-1. We also show that overexpression of alpha-synuclein inhibits protein kinase C activity. The association of alpha-synuclein with BAD and inhibition of protein kinase C suggests that increased expression of alpha-synuclein could be harmful. Consistent with this hypothesis, we observed that overexpression of wild-type alpha-synuclein is toxic, and overexpression of alpha-synuclein containing the A53T or A30P mutations exhibits even greater toxicity. The activity and binding profile of alpha-synuclein suggests that it might act as a protein chaperone and that accumulation of alpha-synuclein could contribute to cell death in neurodegenerative diseases.
Figures




References
-
- Aitken A, Howell S, Jones D, Madrazo J, Martin H, Patel Y, Robinson K. Post-translationally modified 14-3-3 isoforms and inhibition of protein kinase C. Mol Cell Biochem. 1995;149:41–49. - PubMed
-
- Atwood C, Moir R, Huang X, Scarpa R, Bacarra N, Romano D, Hartshorn M, Tanzi R, Bush A. Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem. 1998;273:12817–12826. - PubMed
-
- Behl C, Davis J, Klier F, Schubert D. Amyloid β peptide induces necrosis rather than apoptosis. Brain Res. 1994;645:253–264. - PubMed
-
- Broadie K, Rushton E, Skoulakis E, Davis R. Leonardo, a Drosophila 14-3-3 protein involved in learning, regulates presynaptic function. Neuron. 1997;19:391–402. - PubMed
-
- Clayton D, George J. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21:249–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
