Ca(2+)-permeable AMPA receptors induce phosphorylation of cAMP response element-binding protein through a phosphatidylinositol 3-kinase-dependent stimulation of the mitogen-activated protein kinase signaling cascade in neurons
- PMID: 10407026
- PMCID: PMC6783096
- DOI: 10.1523/JNEUROSCI.19-14-05861.1999
Ca(2+)-permeable AMPA receptors induce phosphorylation of cAMP response element-binding protein through a phosphatidylinositol 3-kinase-dependent stimulation of the mitogen-activated protein kinase signaling cascade in neurons
Abstract
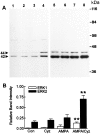
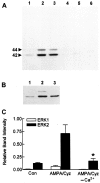
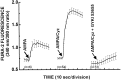
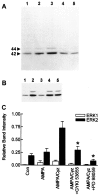
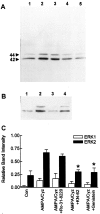
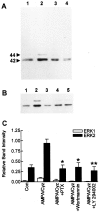
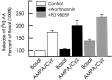
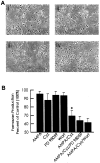
Ca(2+)-permeable AMPA receptors may play a key role during developmental neuroplasticity, learning and memory, and neuronal loss in a number of neuropathologies. However, the intracellular signaling pathways used by AMPA receptors during such processes are not fully understood. The mitogen-activated protein kinase (MAPK) cascade is an attractive target because it has been shown to be involved in gene expression, synaptic plasticity, and neuronal stress. Using primary cultures of mouse striatal neurons and a phosphospecific MAPK antibody we addressed whether AMPA receptors can activate the MAPK cascade. We found that in the presence of cyclothiazide, AMPA caused a robust and direct (no involvement of NMDA receptors or L-type voltage-sensitive Ca(2+) channels) Ca(2+)-dependent activation of MAPK through MAPK kinase (MEK). This activation was blocked by GYKI 53655, a noncompetitive selective antagonist of AMPA receptors. Probing the mechanism of this activation revealed an essential role for phosphatidylinositol 3-kinase (PI 3-kinase) and the involvement of a pertussis toxin (PTX)-sensitive G-protein, a Src family protein tyrosine kinase, and Ca(2+)/calmodulin-dependent kinase II. Similarly, kainate activated MAPK in a PI 3-kinase-dependent manner. AMPA receptor-evoked neuronal death and arachidonic acid mobilization did not appear to involve signaling through the MAPK pathway. However, AMPA receptor stimulation led to a Ca(2+)-dependent phosphorylation of the nuclear transcription factor CREB, which could be prevented by inhibitors of MEK or PI 3-kinase. Our results indicate that Ca(2+)-permeable AMPA receptors transduce signals from the cell surface to the nucleus of neurons through a PI 3-kinase-dependent activation of MAPK. This novel pathway may play a pivotal role in regulating synaptic plasticity in the striatum.
Figures

References
-
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. - PubMed
-
- Bading H, Segal MM, Sucher NJ, Dudek H, Lipton SA, Greenberg ME. N-methyl-d-aspartate receptors are critical for mediating the effects of glutamate on intracellular calcium concentration and immediate early gene expression in cultured hippocampal neurons. Neuroscience. 1995;64:653–664. - PubMed
-
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
