Modulation of glioma cell migration and invasion using Cl(-) and K(+) ion channel blockers
- PMID: 10407033
- PMCID: PMC6783071
- DOI: 10.1523/JNEUROSCI.19-14-05942.1999
Modulation of glioma cell migration and invasion using Cl(-) and K(+) ion channel blockers
Abstract
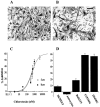
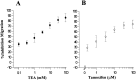
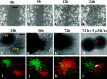
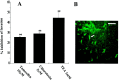
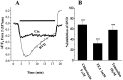
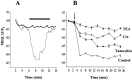
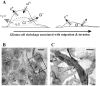
Human malignant gliomas are highly invasive tumors. Mechanisms that allow glioma cells to disseminate, migrating through the narrow extracellular brain spaces are poorly understood. We recently demonstrated expression of large voltage-dependent chloride (Cl(-)) currents, selectively expressed by human glioma cells in vitro and in situ (Ullrich et al., 1998). Currents are sensitive to several Cl(-) channel blockers, including chlorotoxin (Ctx), (Ullrich and Sontheimer; 1996; Ullrich et al; 1996), tetraethylammonium chloride (TEA), and tamoxifen (Ransom and Sontheimer, 1998). Using Transwell migration assays, we show that blockade of glioma Cl(-) channels specifically inhibits tumor cell migration in a dose-dependent manner. Ctx (5 microM), tamoxifen (10 microM), and TEA (1 mM) also prevented invasion of human glioma cells into fetal rat brain aggregates, used as an in vitro model to assess tumor invasiveness. Anion replacement studies suggest that permeation of chloride ions through glioma chloride channel is obligatory for cell migration. Osmotically induced cell swelling and subsequent regulatory volume decrease (RVD) in cultured glioma cells were reversibly prevented by 1 mM TEA, 10 microM tamoxifen, and irreversibly blocked by 5 microM Ctx added to the hypotonic media. Cl(-) fluxes associated with adaptive shape changes elicited by cell swelling and RVD in glioma cells were inhibited by 5 microM Ctx, 10 microM tamoxifen, and 1 mM TEA, as determined using the Cl(-)-sensitive fluorescent dye 6-methoxy-N-ethylquinolinium iodide. Collectively, these data suggest that chloride channels in glioma cells may enable tumor invasiveness, presumably by facilitating cell shape and cell volume changes that are more conducive to migration and invasion.
Figures

References
-
- Andreanski S, Soroceanu L, Flotte ER, Chou J, Markert JM, Gillespie YG, Roizman B, Whitley RJ. Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human malignant brain tumors. Cancer Res. 1997;57:1502–1509. - PubMed
-
- Bernstein J, Goldberg WJ, Laws ER, Conger D, Morreale V, Wood RL. C6 glioma cell invasion and migration of rat brain after neural homografting: ultrastructure. J Neurosurg. 1990;26:622–628. - PubMed
-
- Biwerski J, Verkman AS. Cell permeable fluorescent indicator for cytosolic chloride. Biochemistry. 1991;30:7879–7883. - PubMed
-
- Bjerkvig R, Tonnesen A, Laerum OD, Backlund E. Multicellular tumor spheroids from human gliomas maintained in organ culture. J Neurosurg. 1990;72:463–475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
