Synaptic control of spiking in cerebellar Purkinje cells: dynamic current clamp based on model conductances
- PMID: 10407045
- PMCID: PMC6783079
- DOI: 10.1523/JNEUROSCI.19-14-06090.1999
Synaptic control of spiking in cerebellar Purkinje cells: dynamic current clamp based on model conductances
Abstract
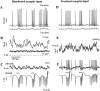
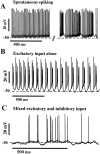
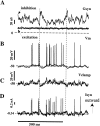
Previous simulations using a realistic model of a cerebellar Purkinje cell suggested that synaptic control of somatic spiking in this cell type is mediated by voltage-gated intrinsic conductances and that inhibitory rather than excitatory synaptic inputs are more influential in controlling spike timing. In this paper, we have tested these predictions physiologically using dynamic current clamping to apply model-derived synaptic conductances to Purkinje cells in vitro. As predicted by the model, this input transformed the in vitro pattern of spiking into a different spike pattern typically observed in vivo. A net inhibitory synaptic current was required to achieve such spiking, indicating the presence of strong intrinsic depolarizing currents. Spike-triggered averaging confirmed that the length of individual intervals between spikes was correlated to the amplitude of the inhibitory conductance but was not influenced by excitatory inputs. Through repeated presentation of identical stimuli, we determined that the output spike rate was very sensitive to the relative balance of excitation and inhibition in the input conductances. In contrast, the accuracy of spike timing was dependent on input amplitude and was independent of spike rate. Thus, information could be encoded in Purkinje cell spiking in a precise spike time code and a rate code at the same time. We conclude that Purkinje cell responses to synaptic input are strongly dependent on active somatic and dendritic properties and that theories of cerebellar function likely need to incorporate single-cell dynamics to a greater degree than is customary.
Figures





References
-
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61.
-
- Barbour B. Synaptic currents evoked in Purkinje cells by stimulating individual granule cells. Neuron. 1993;11:759–769. - PubMed
-
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods. 1994;51:107–116. - PubMed
-
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991;121:101–117. - PubMed
-
- Bhalla US, Bower JM. Exploring parameter space in detailed single neuron models: simulations of the mitral and granule cells of the olfactory bulb. J Neurophysiol. 1993;69:1948–1965. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
