Interdependence of multiple theta generators in the hippocampus: a partial coherence analysis
- PMID: 10407056
- PMCID: PMC6783086
- DOI: 10.1523/JNEUROSCI.19-14-06200.1999
Interdependence of multiple theta generators in the hippocampus: a partial coherence analysis
Abstract
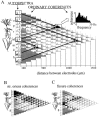
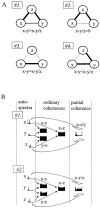
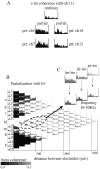
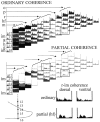
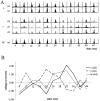
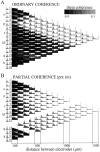
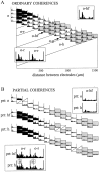
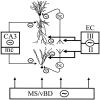
The extracellularly recorded theta oscillation reflects a dynamic interaction of various synaptic and cellular mechanisms. Because the spatially overlapping dipoles responsible for the generation of theta field oscillation may represent different mechanisms, their separation might provide clues with regard to their origin and significance. We used a novel approach, partial coherence analysis, to reveal the various components of the theta rhythm and the relationship among its generators. Hippocampal field activity was recorded by a 16-site silicon probe in the CA1-dentate gyrus axis of the awake rat. Field patterns, recorded from various intrahippocampal or entorhinal cortex sites, were used to remove activity caused by a common source by the partialization procedure. The findings revealed highly coherent coupling between theta signals recorded (1) from the hippocampal fissure and stratum (str.) oriens of the CA1 region and (2) between CA1 stratum radiatum and the dentate molecular layer. The results of partial coherence analysis indicated that rhythmic input from the entorhinal cortex explained theta coherence between signals recorded from the hippocampal fissure and str. oriens but not the coherence between signals derived from str. radiatum and the dentate molecular layer. After bilateral lesions of the entorhinal cortex, all signals recorded from both below and above the CA1 hippocampal pyramidal cell layer became highly coherent. These observations indicate the presence of two, relatively independent, theta generators in the hippocampus, which are mediated by the entorhinal cortex and the CA3-mossy cell recurrent circuitry, respectively. The CA3-mossy cell theta generator is partially suppressed by the dentate gyrus interneuronal output in the intact brain. We suggest that timing of the action potentials of pyramidal cells during the theta cycle is determined by the cooperation between the active CA3 neurons and the entorhinal input.
Figures






References
-
- Alonso A, Garcia-Austt E. Neuronal sources of the theta rhythm in the entorhinal cortex of the rat. II. Phase relations between unit discharges and theta field potentials. Exp Brain Res. 1987;67:502–509. - PubMed
-
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. - PubMed
-
- Andersen P. Interhippocampal impulses. II. Apical dendritic activation of CA1 neurons. Acta Physiol Scand. 1960;48:178–208. - PubMed
-
- Apostol G, Creutzfeldt OD. Crosscorrelation between the activity of septal units and hippocampal EEG during arousal. Brain Res. 1974;67:65–75. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
