High resolution in vivo intra-arterial imaging with optical coherence tomography
- PMID: 10409522
- PMCID: PMC1729132
- DOI: 10.1136/hrt.82.2.128
High resolution in vivo intra-arterial imaging with optical coherence tomography
Abstract
Background: Optical coherence tomography (OCT) is a new method of catheter based micron scale imaging. OCT is analogous to ultrasound, measuring the intensity of backreflected infrared light rather than sound waves.
Objective: To demonstrate the ability of OCT to perform high resolution imaging of arterial tissue in vivo.
Methods: OCT imaging of the abdominal aorta of New Zealand white rabbits was performed using a 2.9 F OCT imaging catheter. Using an ultrashort pulse laser as a light source for imaging, an axial resolution of 10 micrometer was achieved.
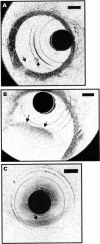
Results: Imaging was performed at 4 frames/second and data were saved in either super VHS or digital format. Saline injections were required during imaging because of the signal attenuation caused by blood. Microstructure was sharply defined within the arterial wall and correlated with histology. Some motion artefacts were noted at 4 frames/second.
Conclusions: In vivo imaging of the rabbit aorta was demonstrated at a source resolution of 10 micrometer, but required the displacement of blood with saline. The high resolution of OCT allows imaging to be performed near the resolution of histopathology, offering the potential to have an impact both on the identification of high risk plaques and the guidance of interventional procedures.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
