An open label, randomised, crossover study comparing sotalol and atenolol in the treatment of symptomatic paroxysmal atrial fibrillation
- PMID: 10409530
- PMCID: PMC1729147
- DOI: 10.1136/hrt.82.2.170
An open label, randomised, crossover study comparing sotalol and atenolol in the treatment of symptomatic paroxysmal atrial fibrillation
Abstract
Objective: To compare sotalol and atenolol in the treatment of symptomatic paroxysmal atrial fibrillation.
Design: Prospective, randomised, open label, crossover study.
Setting: University hospital.
Patients: 47 subjects aged over 50 years were recruited from the hospital outpatient department following ECG documentation of paroxysmal atrial fibrillation that coincided with symptoms. Six patients withdrew and 41 completed the trial.
Interventions: Patients were randomised to one month's treatment with sotalol 80 mg twice daily or atenolol 50 mg once daily. Treatment arms were then crossed over. Patients underwent 72 hour Holter monitoring before randomisation and repeat studies were carried out at the end of both treatment periods. Symptom assessments were completed using linear analogue scales and the Nottingham health profile.
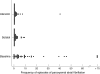
Main outcome measure: Frequency of paroxysmal atrial fibrillation; secondary outcome measures included average and total duration of paroxysmal atrial fibrillation, total ectopic count, and symptom assessments.
Results: A reduction in the number and duration of episodes of paroxysmal atrial fibrillation was noted following treatment with sotalol and atenolol. There was no difference in frequency of paroxysmal atrial fibrillation during treatment with sotalol or atenolol (median difference 0; 95% confidence interval (CI) 0 to 1; p = 0.47). There was no difference in total duration of paroxysmal atrial fibrillation (median difference 0 min; 95% CI -1 to 2; p = 0. 51) or in average duration (median difference 0 min; 95% CI 0 to 1; p = 0.31). No difference was found in total ectopic count between sotalol and atenolol (median difference -123; 95% CI -362 to 135; p = 0.14). Treatments were equally tolerated with no difference in linear analogue scores for symptoms of paroxysmal atrial fibrillation (median difference -5; 95% CI -20 to 5; p = 0.26) or in all categories of the Nottingham health profile.
Conclusions: No difference was found in terms of ECG or symptomatic control of paroxysmal atrial fibrillation between prescribing sotalol 80 mg twice daily and atenolol 50 mg once daily. There was an improvement in paroxysmal atrial fibrillation from baseline following treatment with either sotalol or atenolol.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical