Mutations in elongation factor 1beta, a guanine nucleotide exchange factor, enhance translational fidelity
- PMID: 10409717
- PMCID: PMC84369
- DOI: 10.1128/MCB.19.8.5257
Mutations in elongation factor 1beta, a guanine nucleotide exchange factor, enhance translational fidelity
Abstract
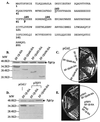
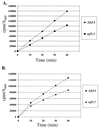
Translation elongation factor 1beta (EF-1beta) is a member of the family of guanine nucleotide exchange factors, proteins whose activities are important for the regulation of G proteins critical to many cellular processes. EF-1beta is a highly conserved protein that catalyzes the exchange of bound GDP for GTP on EF-1alpha, a required step to ensure continued protein synthesis. In this work, we demonstrate that the highly conserved C-terminal region of Saccharomyces cerevisiae EF-1beta is sufficient for normal cell growth. This region of yeast and metazoan EF-1beta and the metazoan EF-1beta-like protein EF-1delta is highly conserved. Human EF-1beta, but not human EF-1delta, is functional in place of yeast EF-1beta, even though both EF-1beta and EF-1delta have previously been shown to have guanine nucleotide exchange activity in vitro. Based on the sequence and functional homology, mutagenesis of two C-terminal residues identical in all EF-1beta protein sequences was performed, resulting in mutants with growth defects and sensitivity to translation inhibitors. These mutants also enhance translational fidelity at nonsense codons, which correlates with a reduction in total protein synthesis. These results indicate the critical function of EF-1beta in regulating EF-1alpha activity, cell growth, translation rates, and translational fidelity.
Figures



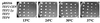

Similar articles
-
The solution structure of the guanine nucleotide exchange domain of human elongation factor 1beta reveals a striking resemblance to that of EF-Ts from Escherichia coli.Structure. 1999 Feb 15;7(2):217-26. doi: 10.1016/s0969-2126(99)80027-6. Structure. 1999. PMID: 10368288
-
Mutation of a conserved CDK site converts a metazoan Elongation Factor 1Bbeta subunit into a replacement for yeast eEF1Balpha.Mol Genet Genomics. 2003 Sep;269(6):776-88. doi: 10.1007/s00438-003-0888-1. Epub 2003 Jul 30. Mol Genet Genomics. 2003. PMID: 12898219
-
Increased expression of Saccharomyces cerevisiae translation elongation factor 1 alpha bypasses the lethality of a TEF5 null allele encoding elongation factor 1 beta.Genetics. 1995 Oct;141(2):481-9. doi: 10.1093/genetics/141.2.481. Genetics. 1995. PMID: 8647386 Free PMC article.
-
Translation elongation factor 3: a fungus-specific translation factor?Mol Microbiol. 1993 Aug;9(3):411-8. doi: 10.1111/j.1365-2958.1993.tb01702.x. Mol Microbiol. 1993. PMID: 8412690 Review.
-
Translational regulation by ABC systems.Res Microbiol. 2001 Apr-May;152(3-4):391-9. doi: 10.1016/s0923-2508(01)01210-4. Res Microbiol. 2001. PMID: 11421286 Review.
Cited by
-
Interplay between GCN2 and GCN4 expression, translation elongation factor 1 mutations and translational fidelity in yeast.Nucleic Acids Res. 2005 Aug 12;33(14):4584-92. doi: 10.1093/nar/gki765. Print 2005. Nucleic Acids Res. 2005. PMID: 16100380 Free PMC article.
-
Differentiating between near- and non-cognate codons in Saccharomyces cerevisiae.PLoS One. 2007 Jun 13;2(6):e517. doi: 10.1371/journal.pone.0000517. PLoS One. 2007. PMID: 17565370 Free PMC article.
-
The C-terminal region of human eukaryotic elongation factor 1Bδ.J Biomol NMR. 2016 Feb;64(2):181-7. doi: 10.1007/s10858-016-0012-6. Epub 2016 Jan 13. J Biomol NMR. 2016. PMID: 26762120 No abstract available.
-
Modulation of efficiency of translation termination in Saccharomyces cerevisiae.Prion. 2014;8(3):247-60. doi: 10.4161/pri.29851. Epub 2014 Nov 1. Prion. 2014. PMID: 25486049 Free PMC article. Review.
-
T-2 toxin induced Salmonella Typhimurium intoxication results in decreased Salmonella numbers in the cecum contents of pigs, despite marked effects on Salmonella-host cell interactions.Vet Res. 2012 Mar 22;43(1):22. doi: 10.1186/1297-9716-43-22. Vet Res. 2012. PMID: 22440148 Free PMC article.
References
-
- Bec G, Kerjan P, Waller J-P. Reconstitution in vitro of the valyl-tRNA synthetase-elongation factor (EF) 1βγδ complex. J Biol Chem. 1994;269:2086–2092. - PubMed
-
- Belfield G P, Tuite M F. Translation elongation factor 3: a fungal-specific translation factor? Mol Microbiol. 1993;9:411–418. - PubMed
-
- Boeke J D, Trueheart J, Natsoulis G, Fink G R. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 1987;154:164–175. - PubMed
-
- Botstein D, Falco S C, Stewart S E, Brennan M, Scherer S, Stinchcomb D T, Struhl K, Davis R W. Sterile host yeast (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979;8:17–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
