B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway
- PMID: 10409722
- PMCID: PMC84374
- DOI: 10.1128/MCB.19.8.5308
B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway
Abstract
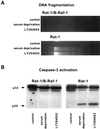
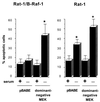
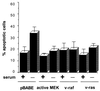
Growth factor-dependent kinases, such as phosphatidylinositol 3-kinase (PI 3-kinase) and Raf kinases, have been implicated in the suppression of apoptosis. We have recently established Rat-1 fibroblast cell lines overexpressing B-Raf, leading to activation of the MEK/Erk mitogen-activated protein kinase pathway. Overexpression of B-Raf confers resistance to apoptosis induced by growth factor withdrawal or PI 3-kinase inhibition. This is accompanied by constitutive activation of Erk without effects on the PI 3-kinase/Akt pathway. The activity of MEK is essential for cell survival mediated by B-Raf overexpression, since either treatment with the specific MEK inhibitor PD98059 or expression of a dominant inhibitory MEK mutant blocks the antiapoptotic activity of B-Raf. Activation of MEK is not only necessary but also sufficient for cell survival because overexpression of constitutively activated MEK, Ras, or Raf-1, like B-Raf, prevents apoptosis after growth factor deprivation. Overexpression of B-Raf did not interfere with the release of cytochrome c from mitochondria after growth factor deprivation. However, the addition of cytochrome c to cytosols of cells overexpressing B-Raf failed to induce caspase activation. It thus appears that the B-Raf/MEK/Erk pathway confers protection against apoptosis at the level of cytosolic caspase activation, downstream of the release of cytochrome c from mitochondria.
Figures
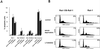





References
-
- Armstrong R C, Aja T, Xiang J, Gaur S, Krebs J F, Hoang K, Bai X, Korsmeyer S J, Karanewsky D S, Fritz L F, Tomaselli K J. Fas-induced activation of the cell death-related protease CPP32 is inhibited by Bcl-2 and by ICE family protease inhibitors. J Biol Chem. 1996;271:16850–16855. - PubMed
-
- Bergmann A, Agapite J, McCall K, Steller H. The Drosophila gene hid is a direct molecular target of ras-dependent survival signaling. Cell. 1998;95:331–341. - PubMed
-
- Berra E, Diaz-Meco M T, Moscat J. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem. 1998;273:10792–10797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
