Dual signaling role of the protein tyrosine phosphatase SHP-2 in regulating expression of acute-phase plasma proteins by interleukin-6 cytokine receptors in hepatic cells
- PMID: 10409724
- PMCID: PMC84376
- DOI: 10.1128/MCB.19.8.5326
Dual signaling role of the protein tyrosine phosphatase SHP-2 in regulating expression of acute-phase plasma proteins by interleukin-6 cytokine receptors in hepatic cells
Abstract
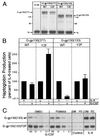
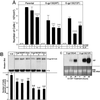
One of the major actions of interleukin-6 (IL-6) is the transcriptional activation of acute-phase plasma proteins (APP) genes in liver cells. Signaling by the IL-6 receptor is mediated through the signal transducing subunit gp130 and involves the activation of Janus-associated kinases (JAKs), signal transducer and activator of transcription 3 (STAT3), and mitogen-activated protein (MAP) kinase. Functional analysis of gp130 in rat hepatoma cells by using transduced chimeric G-CSFR-gp130 receptor constructs demonstrates that SHP-2, the Src homology 2 (SH2) domain-containing protein tyrosine phosphatase, acts as a negative regulator of the JAK/STAT signaling in part by downregulating JAK activity, thereby indirectly moderating the induction of STAT3-dependent APP genes. This study shows that in hepatoma cells, the recruitment and tyrosine phosphorylation of SHP-2, but not SHC, is the primary signaling event associated with the activation of MAP kinases (ERK1/2) by gp130. Overexpression of truncated SHP-2 that lacks Grb2-interacting sites, but not the full-length catalytically inactive SHP-2, reduces ERK activation by IL-6, confirming the signal-mediating role of SHP-2. Activation of ERK1/2 is correlated with induction of the immediate-early response genes. Stimulation of the c-fos, c-jun, and egr-1 genes is essentially absent in cells expressing gp130 with a Y759F mutation, which is unable to recruit SHP-2. Interestingly, both JAK/STAT and SHP-2 pathways regulate the induction of the junB gene. Moreover, disengagement of SHP-2 from gp130 signaling not only enhances APP gene induction but also further reduces cell proliferation, in part correlated with the attenuated expression of immediate-early response genes. These results suggest that IL-6 regulation of APP genes is affected by SHP-2 in two ways: SHP-2 acts as a phosphatase on the JAK/STAT pathway and serves as linker to the MAP kinase pathway, which in turn moderates APP production.
Figures






References
-
- Adachi M, Ishino M, Torigoe T, Minami Y, Matozaki T, Miyazaki T, Taniguchi T, Hinoda Y, Imai K. Interleukin-2 induces tyrosine phosphorylation of SHP-2 through IL-2 receptor beta chain. Oncogene. 1997;14:1629–1633. - PubMed
-
- Auble D T, Brinckerhoff C E. The AP-1 sequence is necessary but not sufficient for phorbol induction of collagenase in fibroblasts. Biochemistry. 1991;30:4629–4635. - PubMed
-
- Baumann H, Prowse K R, Marinokovic S, Won K-A, Jahreis G P. Stimulation of hepatic acute phase response by cytokines and glucocorticoids. Ann N Y Acad Sci. 1989;557:280–295. - PubMed
-
- Baumann H, Gearing D, Ziegler S F. Signaling by the cytoplasmic domain of hematopoietin receptors involves two distinguishable mechanisms in hepatic cells. J Biol Chem. 1994;269:16297–16304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
