Kinase suppressor of Ras forms a multiprotein signaling complex and modulates MEK localization
- PMID: 10409742
- PMCID: PMC84397
- DOI: 10.1128/MCB.19.8.5523
Kinase suppressor of Ras forms a multiprotein signaling complex and modulates MEK localization
Abstract
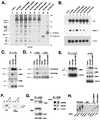
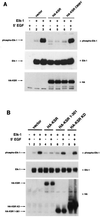
Genetic screens for modifiers of activated Ras phenotypes have identified a novel protein, kinase suppressor of Ras (KSR), which shares significant sequence homology with Raf family protein kinases. Studies using Drosophila melanogaster and Caenorhabditis elegans predict that KSR positively regulates Ras signaling; however, the function of mammalian KSR is not well understood. We show here that two predicted kinase-dead mutants of KSR retain the ability to complement ksr-1 loss-of-function alleles in C. elegans, suggesting that KSR may have physiological, kinase-independent functions. Furthermore, we observe that murine KSR forms a multimolecular signaling complex in human embryonic kidney 293T cells composed of HSP90, HSP70, HSP68, p50(CDC37), MEK1, MEK2, 14-3-3, and several other, unidentified proteins. Treatment of cells with geldanamycin, an inhibitor of HSP90, decreases the half-life of KSR, suggesting that HSPs may serve to stabilize KSR. Both nematode and mammalian KSRs are capable of binding to MEKs, and three-point mutants of KSR, corresponding to C. elegans loss-of-function alleles, are specifically compromised in MEK binding. KSR did not alter MEK activity or activation. However, KSR-MEK binding shifts the apparent molecular mass of MEK from 44 to >700 kDa, and this results in the appearance of MEK in membrane-associated fractions. Together, these results suggest that KSR may act as a scaffolding protein for the Ras-mitogen-activated protein kinase pathway.
Figures




References
-
- Beitel G J, Clark S G, Horvitz H R. Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature. 1990;348:503–509. - PubMed
-
- Choi K Y, Satterberg B, Lyons D M, Elion E A. Ste5 tethers multiple protein kinases in the MAP kinase cascade required for mating in S. cerevisiae. Cell. 1994;78:499–512. - PubMed
-
- Cutforth T, Rubin G M. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell. 1994;77:1027–1036. - PubMed
-
- Dang A, Frost J A, Cobb M H. The MEK1 proline-rich insert is required for efficient activation of the mitogen-activated protein kinases ERK1 and ERK2 in mammalian cells. J Biol Chem. 1998;273:19909–19913. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
