Safety and antitumor activity of recombinant soluble Apo2 ligand
- PMID: 10411544
- PMCID: PMC408479
- DOI: 10.1172/JCI6926
Safety and antitumor activity of recombinant soluble Apo2 ligand
Abstract
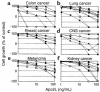
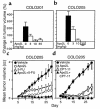
TNF and Fas ligand induce apoptosis in tumor cells; however, their severe toxicity toward normal tissues hampers their application to cancer therapy. Apo2 ligand (Apo2L, or TRAIL) is a related molecule that triggers tumor cell apoptosis. Apo2L mRNA is expressed in many tissues, suggesting that the ligand may be nontoxic to normal cells. To investigate Apo2L's therapeutic potential, we generated in bacteria a potently active soluble version of the native human protein. Several normal cell types were resistant in vitro to apoptosis induction by Apo2L. Repeated intravenous injections of Apo2L in nonhuman primates did not cause detectable toxicity to tissues and organs examined. Apo2L exerted cytostatic or cytotoxic effects in vitro on 32 of 39 cell lines from colon, lung, breast, kidney, brain, and skin cancer. Treatment of athymic mice with Apo2L shortly after tumor xenograft injection markedly reduced tumor incidence. Apo2L treatment of mice bearing solid tumors induced tumor cell apoptosis, suppressed tumor progression, and improved survival. Apo2L cooperated synergistically with the chemotherapeutic drugs 5-fluorouracil or CPT-11, causing substantial tumor regression or complete tumor ablation. Thus, Apo2L may have potent anticancer activity without significant toxicity toward normal tissues.
Figures





References
-
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. - PubMed
-
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. - PubMed
-
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. - PubMed
-
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. - PubMed
-
- Tartaglia L, Goeddel D. Two TNF receptors. Immunol Today. 1992;13:151–153. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

