Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells
- PMID: 10411546
- PMCID: PMC408478
- DOI: 10.1172/JCI6909
Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells
Abstract
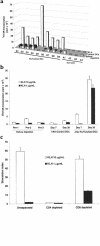
Dendritic cells (DCs) are potent antigen-presenting cells that initiate protective T-cell immunity in mice. To study the immunogenicity of DCs in humans, we injected 9 healthy subjects subcutaneously with a control injection of autologous monocyte-derived, mature DCs, followed 4-6 weeks later by DCs pulsed with keyhole limpet hemocyanin (KLH), HLA-A*0201-positive restricted influenza matrix peptide (MP), and tetanus toxoid (TT). Four more subjects received these antigens without DCs. Injection of unpulsed DCs, or antigens alone, failed to immunize. Priming of CD4(+) T cells to KLH was observed in all 9 subjects injected with KLH-pulsed DCs, and boosting of TT-specific T-cell immunity was seen in 5 of 6 subjects injected with TT-pulsed DCs. Injection of antigen-pulsed DCs led to a severalfold increase in freshly isolated MP-specific, IFN-gamma-secreting CD8(+) T cells in all 6 HLA-A*0201-positive subjects, as early as 7 days after injection. When T cells were boosted in culture, there was an increase in MHC tetramer-binding cells and cytotoxic T cells after DC vaccination. These data provide the first controlled evidence of the immunogenicity of DCs in humans, and demonstrate that a single injection of mature DCs rapidly expands T-cell immunity.
Figures
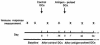




References
-
- Raychaudhuri S, Rock KL. Fully mobilizing host defense: building better vaccines. Nat Biotechnol. 1998;16:1025–1031. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. - PubMed
-
- Mayordomo JI, et al. Bone marrow–derived dendritic cells serve as potent adjuvants for peptide-based antitumor vaccines. Stem Cells. 1997;15:94–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

