Guest exchange in an encapsulation complex: a supramolecular substitution reaction
- PMID: 10411877
- PMCID: PMC17519
- DOI: 10.1073/pnas.96.15.8344
Guest exchange in an encapsulation complex: a supramolecular substitution reaction
Abstract
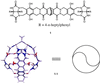
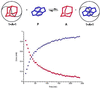
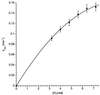
Encapsulation complexes are reversibly formed assemblies in which small molecule guests are completely surrounded by large molecule hosts. The assemblies are held together by weak intermolecular forces and are dynamic: they form and dissipate on time scales ranging from milliseconds to days-long enough for many interactions, even reactions, to take place within them. Little information is available on the exchange process, how guests get in and out of these complexes. Here we report that these events can be slow enough for conventional kinetic studies, and reactive intermediates can be detected. Guest exchange has much in common with familiar chemical substitution reactions, but differs in some respects: no covalent bonds are made or broken, the substrate is an assembly rather than a single molecule, and at least four molecules are involved in multiple rate-determining steps.
Figures
References
-
- Sherman J C, Cram D J. J Am Chem Soc. 1989;111:4527–4528.
-
- Collet A, Dutsata J P, Lozach B, Canceill J. In: Chemistry I: Directed Synthesis and Molecular Recognition. Weber E, editor. Berlin: Springer; 1993. pp. 104–129.
-
- Cram D J, Tanner M E, Thomas R. Angew Chem Int Ed Engl. 1991;30:1024–1027.
-
- Timmerman P, Verboom W, van Vegge F C J M, van Duynhove J P M, Reinhoudt D N. Angew Chem Int Ed Engl. 1994;33:2345–2348.
-
- Costante-Crassous J, Marrone T J, Briggs J M, McCammon J A, Collet A. J Am Chem Soc. 1997;119:3818–3823.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

