A novel function for transglutaminase 1: attachment of long-chain omega-hydroxyceramides to involucrin by ester bond formation
- PMID: 10411887
- PMCID: PMC17528
- DOI: 10.1073/pnas.96.15.8402
A novel function for transglutaminase 1: attachment of long-chain omega-hydroxyceramides to involucrin by ester bond formation
Abstract
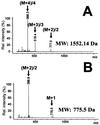
Transglutaminases (TGases) are defined as enzymes capable of forming isopeptide bonds by transfer of an amine onto glutaminyl residues of a protein. Here we show that the membrane-bound form of the TGase 1 enzyme can also form ester bonds between specific glutaminyl residues of human involucrin and a synthetic analog of epidermal specific omega-hydroxyceramides. The formation of a approximately 5-nm-thick lipid envelope on the surface of epidermal keratinocytes is an important component of normal barrier function. The lipid envelope consists of omega-hydroxyceramides covalently linked by ester bonds to cornified envelope proteins, most abundantly to involucrin. We synthesized an analog of natural omega-hydroxyceramides N-[16-(16-hydroxyhexadecyl)oxypalmitoyl]sphingosine (lipid Z). When recombinant human TGase 1 and involucrin were reacted on the surface of synthetic lipid vesicles containing lipid Z, lipid Z was attached to involucrin and formed saponifiable protein-lipid adducts. By mass spectroscopy and sequencing of tryptic lipopeptides, the ester linkage formation used involucrin glutamine residues 107, 118, 122, 133, and 496 by converting the gamma-carboxamido groups to lipid esters. Several of these residues have been found previously to be attached to ceramides in vivo. Mass spectrometric analysis after acetonide derivatization also revealed that ester formation involved primarily the omega-hydroxyl group of lipid Z. Our data reveal a dual role for TGase 1 in epidermal barrier formation and provide insights into the pathophysiology of lamellar ichthyosis resulting from defects of TGase 1 enzyme.
Figures




Similar articles
-
Cholesterol 3-sulfate interferes with cornified envelope assembly by diverting transglutaminase 1 activity from the formation of cross-links and esters to the hydrolysis of glutamine.J Biol Chem. 2000 Jan 28;275(4):2636-46. doi: 10.1074/jbc.275.4.2636. J Biol Chem. 2000. PMID: 10644724
-
Bricks and mortar of the epidermal barrier.Exp Mol Med. 1999 Mar 31;31(1):5-19. doi: 10.1038/emm.1999.2. Exp Mol Med. 1999. PMID: 10231017 Review.
-
Involucrin cross-linking by transglutaminase 1. Binding to membranes directs residue specificity.J Biol Chem. 1999 Apr 16;274(16):11013-21. doi: 10.1074/jbc.274.16.11013. J Biol Chem. 1999. PMID: 10196183
-
The glutamine residues reactive in transglutaminase-catalyzed cross-linking of involucrin.J Biol Chem. 1988 Dec 5;263(34):18093-8. J Biol Chem. 1988. PMID: 2461365
-
Lipid Metabolic Events Underlying the Formation of the Corneocyte Lipid Envelope.Skin Pharmacol Physiol. 2021;34(1):38-50. doi: 10.1159/000513261. Epub 2021 Feb 10. Skin Pharmacol Physiol. 2021. PMID: 33567435 Review.
Cited by
-
Overproduction of pro-transglutaminase from Streptomyces hygroscopicus in Yarrowia lipolytica and its biochemical characterization.BMC Biotechnol. 2015 Aug 14;15:75. doi: 10.1186/s12896-015-0193-1. BMC Biotechnol. 2015. PMID: 26272462 Free PMC article.
-
Measurement of trihydroxy-linoleic acids in stratum corneum by tape-stripping: Possible biomarker of barrier function in atopic dermatitis.PLoS One. 2019 Jan 4;14(1):e0210013. doi: 10.1371/journal.pone.0210013. eCollection 2019. PLoS One. 2019. PMID: 30608955 Free PMC article.
-
Development of ichthyosiform skin compensates for defective permeability barrier function in mice lacking transglutaminase 1.J Clin Invest. 2002 Jan;109(2):243-50. doi: 10.1172/JCI13563. J Clin Invest. 2002. PMID: 11805136 Free PMC article.
-
SDR9C7 catalyzes critical dehydrogenation of acylceramides for skin barrier formation.J Clin Invest. 2020 Feb 3;130(2):890-903. doi: 10.1172/JCI130675. J Clin Invest. 2020. PMID: 31671075 Free PMC article.
-
E-cadherin and transglutaminase-1 epithelial barrier restoration precedes type IV collagen basement membrane reconstruction following vocal fold mucosal injury.Cells Tissues Organs. 2011;193(3):158-69. doi: 10.1159/000318605. Epub 2010 Oct 20. Cells Tissues Organs. 2011. PMID: 20962500 Free PMC article.
References
-
- Downing D T, Stewart M E, Wertz P W, Strauss J S. In: Dermatology in General Medicine. Fitzpatrick T B, Eisen A Z, Wolff K, Freedberg I M, Austen K F, editors. New York: McGraw–Hill; 1993. pp. 210–221.
-
- Ponec M. In: The Keratinocyte Handbook. Leigh I M, Lane E, Watt F M, editors. Cambridge, U.K.: Cambridge Univ. Press; 1994. pp. 351–363.
-
- Elias P M, Menon G K. In: Skin Lipids, Advances in Lipid Research. Elias P M, editor. Vol. 24. San Diego: Academic; 1991. pp. 1–26. - PubMed
-
- Wertz P W. Experientia Suppl. 1997;78:227–237. - PubMed
-
- Wertz P W, Downing D T. In: Physiology, Biochemistry and Molecular Biology of the Skin. Goldsmith L A, editor. Vol. 1. Oxford: Oxford Univ. Press; 1991. pp. 205–236.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

