In vivo proliferation of naïve and memory influenza-specific CD8(+) T cells
- PMID: 10411921
- PMCID: PMC17562
- DOI: 10.1073/pnas.96.15.8597
In vivo proliferation of naïve and memory influenza-specific CD8(+) T cells
Abstract
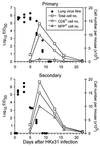
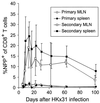
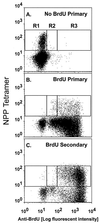
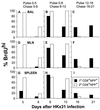
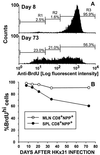
The virus-specific CD8(+) T cell response has been analyzed through the development, effector, and recovery phases of primary and secondary influenza pneumonia. Apparently, most, if not all, memory T cells expressing clonotypic receptors that bind a tetrameric complex of influenza nucleoprotein (NP)(366-374) peptide+H-2D(b) (NPP) are induced to divide during the course of this localized respiratory infection. The replicative phase of the recall response ends about the time that virus can no longer be recovered from the lung, whereas some primary CD8(+)NPP(+) T cells may proliferate for a few more days. The greatly expanded population of CD8(+)NPP(+) memory T cells in the lymphoid tissue of secondarily challenged mice declines progressively in mean prevalence over the ensuing 100 days, despite the fact that at least some of these lymphocytes continue to cycle. The recall of cell-mediated immunity thus is characterized by massive proliferation of the antigen-specific CD8(+) set, whereas the extent of lymphocyte turnover in the absence of cognate peptide is variable, at a low level, and can be influenced by intercurrent infection.
Figures
Similar articles
-
Concurrent naive and memory CD8(+) T cell responses to an influenza A virus.J Immunol. 2001 Sep 1;167(5):2753-8. doi: 10.4049/jimmunol.167.5.2753. J Immunol. 2001. PMID: 11509619
-
Antigen-specific CD8(+) T cells persist in the upper respiratory tract following influenza virus infection.J Immunol. 2001 Sep 15;167(6):3293-9. doi: 10.4049/jimmunol.167.6.3293. J Immunol. 2001. PMID: 11544317
-
Measuring the diaspora for virus-specific CD8+ T cells.Proc Natl Acad Sci U S A. 2001 May 22;98(11):6313-8. doi: 10.1073/pnas.101132698. Epub 2001 May 8. Proc Natl Acad Sci U S A. 2001. PMID: 11344265 Free PMC article.
-
A previously unrecognized H-2D(b)-restricted peptide prominent in the primary influenza A virus-specific CD8(+) T-cell response is much less apparent following secondary challenge.J Virol. 2000 Apr;74(8):3486-93. doi: 10.1128/jvi.74.8.3486-3493.2000. J Virol. 2000. PMID: 10729122 Free PMC article.
-
Establishment and recall of CD8+ T-cell memory in a model of localized transient infection.Immunol Rev. 2006 Jun;211:133-45. doi: 10.1111/j.0105-2896.2006.00386.x. Immunol Rev. 2006. PMID: 16824123 Review.
Cited by
-
A virus-specific CD8+ T cell immunodominance hierarchy determined by antigen dose and precursor frequencies.Proc Natl Acad Sci U S A. 2006 Jan 24;103(4):994-9. doi: 10.1073/pnas.0510429103. Epub 2006 Jan 17. Proc Natl Acad Sci U S A. 2006. PMID: 16418289 Free PMC article.
-
Postexposure vaccination massively increases the prevalence of gamma-herpesvirus-specific CD8+ T cells but confers minimal survival advantage on CD4-deficient mice.Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2725-30. doi: 10.1073/pnas.040575197. Proc Natl Acad Sci U S A. 2000. PMID: 10694575 Free PMC article.
-
The virus-immunity ecosystem.Arch Virol Suppl. 2005;(19):17-32. doi: 10.1007/3-211-29981-5_3. Arch Virol Suppl. 2005. PMID: 16355866 Free PMC article. Review.
-
Innate immune control and regulation of influenza virus infections.J Leukoc Biol. 2009 Oct;86(4):803-12. doi: 10.1189/jlb.0509368. Epub 2009 Jul 30. J Leukoc Biol. 2009. PMID: 19643736 Free PMC article. Review.
-
Consequences of immunodominant epitope deletion for minor influenza virus-specific CD8+-T-cell responses.J Virol. 2005 Apr;79(7):4329-39. doi: 10.1128/JVI.79.7.4329-4339.2005. J Virol. 2005. PMID: 15767433 Free PMC article.
References
-
- Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Science. 1996;274:94–96. - PubMed
-
- Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Immunity. 1998;8:177–187. - PubMed
-
- Flynn K J, Belz G T, Altman J D, Ahmed R, Woodland D L, Doherty P C. Immunity. 1998;8:683–691. - PubMed
-
- Doherty P C, Topham D J, Tripp R A. Immunol Rev. 1996;150:23–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

