The lack of consensus for I-A(g7)-peptide binding motifs: is there a requirement for anchor amino acid side chains?
- PMID: 10411925
- PMCID: PMC17566
- DOI: 10.1073/pnas.96.15.8621
The lack of consensus for I-A(g7)-peptide binding motifs: is there a requirement for anchor amino acid side chains?
Abstract
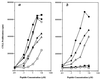
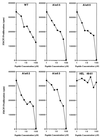
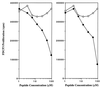
We discuss here the problems in identifying sequence motifs of peptides that bind to I-A(g7), the class II histocompatibility molecule of NOD diabetic mice. We present studies that indicate a minor contribution of amino acid side chains for binding. A peptide from the Ealpha chain binds to I-A(g7) molecules and is recognized by CD4 T cells. By producing single-residue mutations we identified four residues that were considered to contact the T cell receptor. No residue was found to be essential for binding to I-A(g7): a peptide that contained the T cell contact residues, on a backbone of alanines, bound to I-A(g7) and stimulated the T cells. We conclude that peptides can bind to I-A(g7) without the requirement for residues with prominent side chains to anchor them.
Figures

Similar articles
-
IL-12 administration reveals diabetogenic T cells in genetically resistant I-Ealpha-transgenic nonobese diabetic mice: resistance to autoimmune diabetes is associated with binding of Ealpha-derived peptides to the I-A(g7) molecule.J Immunol. 2001 Oct 1;167(7):4104-14. doi: 10.4049/jimmunol.167.7.4104. J Immunol. 2001. PMID: 11564833
-
Functional consequences of the binding of MHC class II-derived peptides to MHC class II.Int Immunol. 1996 Dec;8(12):1857-65. doi: 10.1093/intimm/8.12.1857. Int Immunol. 1996. PMID: 8982770
-
Recognition of core and flanking amino acids of MHC class II-bound peptides by the T cell receptor.Eur J Immunol. 2002 Sep;32(9):2510-20. doi: 10.1002/1521-4141(200209)32:9<2510::AID-IMMU2510>3.0.CO;2-Q. Eur J Immunol. 2002. PMID: 12207335
-
Dissection of the Hb(64-76) determinant reveals that the T cell receptor may have the capacity to differentially signal.Adv Exp Med Biol. 1992;323:17-21. doi: 10.1007/978-1-4615-3396-2_3. Adv Exp Med Biol. 1992. PMID: 1485563 Review. No abstract available.
-
Structural characteristics of peptides required for their interaction with IAd.Ann N Y Acad Sci. 1988;546:72-9. doi: 10.1111/j.1749-6632.1988.tb21621.x. Ann N Y Acad Sci. 1988. PMID: 3073701 Review. No abstract available.
Cited by
-
Antigen presentation: lysoyme, autoimmune diabetes, and Listeria--what do they have in common?Immunol Res. 2005;32(1-3):267-92. doi: 10.1385/IR:32:1-3:267. Immunol Res. 2005. PMID: 16106079 Review.
-
Predicting peptides binding to MHC class II molecules using multi-objective evolutionary algorithms.BMC Bioinformatics. 2007 Nov 22;8:459. doi: 10.1186/1471-2105-8-459. BMC Bioinformatics. 2007. PMID: 18031584 Free PMC article.
References
-
- Reich E-P, von Grafenstein H, Barlow A, Swenson K E, Williams K, Janeway C A., Jr J Immunol. 1994;152:2279–2288. - PubMed
-
- Amor S, O’Neill J K, Morris M M, Smith R M, Wraith D C, Groome N, Travers P J, Baker D. J Immunol. 1996;156:3000–3008. - PubMed
-
- Reizis B, Eisenstein M, Bocková J, Könen-Waisman S, Mor F, Elias D, Cohen I R. Int Immunol. 1997;9:43–51. - PubMed
-
- Chao C C, McDevitt H O. Immunogenetics. 1997;46:29–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

