The lack of consensus for I-A(g7)-peptide binding motifs: is there a requirement for anchor amino acid side chains?
- PMID: 10411925
- PMCID: PMC17566
- DOI: 10.1073/pnas.96.15.8621
The lack of consensus for I-A(g7)-peptide binding motifs: is there a requirement for anchor amino acid side chains?
Abstract
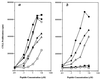
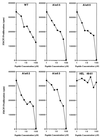
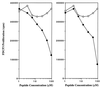
We discuss here the problems in identifying sequence motifs of peptides that bind to I-A(g7), the class II histocompatibility molecule of NOD diabetic mice. We present studies that indicate a minor contribution of amino acid side chains for binding. A peptide from the Ealpha chain binds to I-A(g7) molecules and is recognized by CD4 T cells. By producing single-residue mutations we identified four residues that were considered to contact the T cell receptor. No residue was found to be essential for binding to I-A(g7): a peptide that contained the T cell contact residues, on a backbone of alanines, bound to I-A(g7) and stimulated the T cells. We conclude that peptides can bind to I-A(g7) without the requirement for residues with prominent side chains to anchor them.
Figures

References
-
- Reich E-P, von Grafenstein H, Barlow A, Swenson K E, Williams K, Janeway C A., Jr J Immunol. 1994;152:2279–2288. - PubMed
-
- Amor S, O’Neill J K, Morris M M, Smith R M, Wraith D C, Groome N, Travers P J, Baker D. J Immunol. 1996;156:3000–3008. - PubMed
-
- Reizis B, Eisenstein M, Bocková J, Könen-Waisman S, Mor F, Elias D, Cohen I R. Int Immunol. 1997;9:43–51. - PubMed
-
- Chao C C, McDevitt H O. Immunogenetics. 1997;46:29–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

