Cancer dormancy and cell signaling: induction of p21(waf1) initiated by membrane IgM engagement increases survival of B lymphoma cells
- PMID: 10411940
- PMCID: PMC17581
- DOI: 10.1073/pnas.96.15.8711
Cancer dormancy and cell signaling: induction of p21(waf1) initiated by membrane IgM engagement increases survival of B lymphoma cells
Abstract
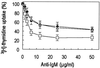
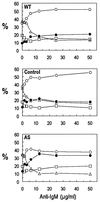
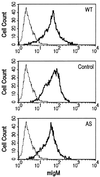
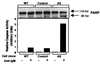
The p21(WAF1) (p21) cyclin-dependent kinase inhibitor plays a major role in regulating cell cycle arrest. It was recently reported that the p53-independent elevation of p21 protein levels is essential in mediating the G(1) arrest resulting from signal transduction events initiated by the crosslinking of membrane IgM on Daudi Burkitt lymphoma cells. Although the role of p21 in cell cycle regulation is well documented, there is little information concerning its role in antibody-mediated apoptosis. In the present study, we examined the involvement of p21 in the regulation of apoptosis by suppressing its induction in anti-IgM-treated Daudi cells through a p21 antisense expression construct approach. Reduction in induced p21 protein levels resulted in diminished G(1) arrest and increased apoptosis. The increased susceptibility to anti-IgM-mediated apoptosis was associated with increased caspase-3-like activity and poly-(ADP)ribose polymerase cleavage. These data suggest that p21 may directly interfere with the caspase cascade, thus playing a dual role in regulating both cell cycle progression and apoptosis.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

