Transgenic mice expressing a Huntington's disease mutation are resistant to quinolinic acid-induced striatal excitotoxicity
- PMID: 10411943
- PMCID: PMC17584
- DOI: 10.1073/pnas.96.15.8727
Transgenic mice expressing a Huntington's disease mutation are resistant to quinolinic acid-induced striatal excitotoxicity
Abstract
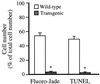
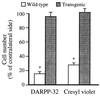
Huntington's disease (HD) is a hereditary neurodegenerative disorder presenting with chorea, dementia, and extensive striatal neuronal death. The mechanism through which the widely expressed mutant HD gene mediates a slowly progressing striatal neurotoxicity is unknown. Glutamate receptor-mediated excitotoxicity has been hypothesized to contribute to the pathogenesis of HD. Here we show that transgenic HD mice expressing exon 1 of a human HD gene with an expanded number of CAG repeats (line R6/1) are strongly protected from acute striatal excitotoxic lesions. Intrastriatal infusions of the N-methyl-D-aspartate (NMDA) receptor agonist quinolinic acid caused massive striatal neuronal death in wild-type mice, but no damage in transgenic HD littermates. The remarkable neuroprotection in transgenic HD mice occurred at a stage when they had not developed any neurological symptoms caused by the mutant HD gene. At this stage there was no change in the number of striatal neurons and astrocytes in untreated R6/1 mice, although the striatal volume was decreased by 17%. Moreover, transgenic HD mice had normal striatal levels of NMDA receptors, calbindin D28k (calcium buffer), superoxide dismutase activity (antioxidant enzyme), Bcl-2 (anti-apoptotic protein), heat shock protein 70 (stress-induced anti-apoptotic protein), and citrate synthase activity (mitochondrial enzyme). We propose that the presence of exon 1 of the mutant HD gene induces profound changes in striatal neurons that render these cells resistant to excessive NMDA receptor activation.
Figures



Similar articles
-
Partial resistance to malonate-induced striatal cell death in transgenic mouse models of Huntington's disease is dependent on age and CAG repeat length.J Neurochem. 2001 Aug;78(4):694-703. doi: 10.1046/j.1471-4159.2001.00482.x. J Neurochem. 2001. PMID: 11520890
-
Resistance to NMDA toxicity correlates with appearance of nuclear inclusions, behavioural deficits and changes in calcium homeostasis in mice transgenic for exon 1 of the huntington gene.Eur J Neurosci. 2001 Nov;14(9):1492-504. doi: 10.1046/j.0953-816x.2001.01767.x. Eur J Neurosci. 2001. PMID: 11722611
-
Mice transgenic for exon 1 of the Huntington's disease gene display reduced striatal sensitivity to neurotoxicity induced by dopamine and 6-hydroxydopamine.Eur J Neurosci. 2001 Nov;14(9):1425-35. doi: 10.1046/j.0953-816x.2001.01765.x. Eur J Neurosci. 2001. PMID: 11722604
-
Excitotoxic neuronal death and the pathogenesis of Huntington's disease.Arch Med Res. 2008 Apr;39(3):265-76. doi: 10.1016/j.arcmed.2007.11.011. Arch Med Res. 2008. PMID: 18279698 Review.
-
The quinolinic acid hypothesis in Huntington's chorea.J Neurol Sci. 1990 Jan;95(1):29-38. doi: 10.1016/0022-510x(90)90114-3. J Neurol Sci. 1990. PMID: 2159984 Review.
Cited by
-
Age-Dependent Resistance to Excitotoxicity in Htt CAG140 Mice and the Effect of Strain Background.J Huntingtons Dis. 2012;1(2):221-41. doi: 10.3233/JHD-129005. J Huntingtons Dis. 2012. PMID: 23833693 Free PMC article.
-
Of mice and men: solving the molecular mysteries of Huntington's disease.J Anat. 2000 May;196 ( Pt 4)(Pt 4):617-28. doi: 10.1046/j.1469-7580.2000.19640617.x. J Anat. 2000. PMID: 10923992 Free PMC article. Review.
-
Proteasome activator enhances survival of Huntington's disease neuronal model cells.PLoS One. 2007 Feb 28;2(2):e238. doi: 10.1371/journal.pone.0000238. PLoS One. 2007. PMID: 17327906 Free PMC article.
-
Selective Neuronal Death in Neurodegenerative Diseases: The Ongoing Mystery.Yale J Biol Med. 2019 Dec 20;92(4):695-705. eCollection 2019 Dec. Yale J Biol Med. 2019. PMID: 31866784 Free PMC article. Review.
-
Wild-type huntingtin ameliorates striatal neuronal atrophy but does not prevent other abnormalities in the YAC128 mouse model of Huntington disease.BMC Neurosci. 2006 Dec 5;7:80. doi: 10.1186/1471-2202-7-80. BMC Neurosci. 2006. PMID: 17147801 Free PMC article.
References
-
- Harper P S. Huntington’s Disease. London: Saunders; 1996.
-
- Vonsattel J P, Myers R H, Stevens T J, Ferrante R J, Bird E D, Richardson E P., Jr J Neuropathol Exp Neurol. 1985;44:559–577. - PubMed
-
- Hedreen J C, Folstein S E. J Neuropathol Exp Neurol. 1995;54:105–120. - PubMed
-
- The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–983. - PubMed
-
- MacDonald M E, Gusella J F. Curr Opin Neurobiol. 1996;6:638–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

