Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons
- PMID: 10411946
- PMCID: PMC17587
- DOI: 10.1073/pnas.96.15.8745
Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons
Abstract
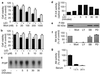
This report describes a modulatory action of lithium and glutamate on the activity of serine/threonine kinase Akt-1. Lithium is most commonly used to treat bipolar disorder, but the mechanism of its therapeutic action remains unknown. We have recently demonstrated that lithium protects against glutamate-induced excitotoxicity in cultured brain neurons and in an animal model of cerebral ischemia. This study was undertaken to investigate the role of Akt-1, activated by the phosphatidylinositol 3-kinase (PI 3-K) signaling pathway, in mediating glutamate excitotoxicity and lithium protection in cerebellar granule cells. High levels of phosphorylation and activity of Akt-1 were detected in cerebellar neurons cultured in the presence of serum. Protracted treatment with selective PI 3-K inhibitors, wortmannin and LY294002, abolished Akt-1 activity and induced neuronal death that could be reduced by long-term lithium pretreatment. Exposure of cells to glutamate induced a rapid and reversible loss of Akt-1 phosphorylation and kinase activity. These effects were closely correlated with excitotoxicity and caspase 3 activation and were prevented by phosphatase inhibitors, okadaic acid and caliculin A. Long-term lithium pretreatment suppressed glutamate-induced loss of Akt-1 activity and accelerated its recovery toward the control levels. Lithium treatment alone induced rapid increase in PI 3-K activity, and Akt-1 phosphorylation with accompanying kinase activation, which was blocked by PI 3-K inhibitors. Lithium also increased the phosphorylation of glycogen synthase kinase-3 (GSK-3), a downstream physiological target of Akt. Thus, modulation of Akt-1 activity appears to play a key role in the mechanism of glutamate excitotoxicity and lithium neuroprotection.
Figures


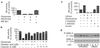
Similar articles
-
Minocycline prevents glutamate-induced apoptosis of cerebellar granule neurons by differential regulation of p38 and Akt pathways.J Neurochem. 2004 Dec;91(5):1219-30. doi: 10.1111/j.1471-4159.2004.02796.x. J Neurochem. 2004. PMID: 15569265
-
Neuroprotection by scatter factor/hepatocyte growth factor and FGF-1 in cerebellar granule neurons is phosphatidylinositol 3-kinase/akt-dependent and MAPK/CREB-independent.J Neurochem. 2002 Apr;81(2):365-78. doi: 10.1046/j.1471-4159.2002.00837.x. J Neurochem. 2002. PMID: 12064484
-
Co-activation of the phosphatidylinositol-3-kinase/Akt signaling pathway by N-methyl-D-aspartate and TrkB receptors in cerebellar granule cell neurons.Amino Acids. 2002;23(1-3):11-7. doi: 10.1007/s00726-001-0103-9. Amino Acids. 2002. PMID: 12373512
-
Neuroprotective and neurotrophic actions of the mood stabilizer lithium: can it be used to treat neurodegenerative diseases?Crit Rev Neurobiol. 2004;16(1-2):83-90. doi: 10.1615/critrevneurobiol.v16.i12.90. Crit Rev Neurobiol. 2004. PMID: 15581403 Review.
-
Neuroprotective effects of lithium in cultured cells and animal models of diseases.Bipolar Disord. 2002 Apr;4(2):129-36. doi: 10.1034/j.1399-5618.2002.01179.x. Bipolar Disord. 2002. PMID: 12071510 Review.
Cited by
-
PFC mTOR signaling as a biological signature for cognitive deficits in bipolar disorder without psychosis.Cell Rep Med. 2021 May 18;2(5):100282. doi: 10.1016/j.xcrm.2021.100282. eCollection 2021 May 18. Cell Rep Med. 2021. PMID: 34095884 Free PMC article.
-
Recent Advances on the Role of GSK3β in the Pathogenesis of Amyotrophic Lateral Sclerosis.Brain Sci. 2020 Sep 26;10(10):675. doi: 10.3390/brainsci10100675. Brain Sci. 2020. PMID: 32993098 Free PMC article. Review.
-
Abnormal development of NG2+PDGFR-α+ neural progenitor cells leads to neonatal hydrocephalus in a ciliopathy mouse model.Nat Med. 2012 Dec;18(12):1797-804. doi: 10.1038/nm.2996. Epub 2012 Nov 18. Nat Med. 2012. PMID: 23160237 Free PMC article.
-
A small molecule transcription factor EB activator ameliorates beta-amyloid precursor protein and Tau pathology in Alzheimer's disease models.Aging Cell. 2020 Feb;19(2):e13069. doi: 10.1111/acel.13069. Epub 2019 Dec 19. Aging Cell. 2020. PMID: 31858697 Free PMC article.
-
Inhibition of GSK-3β on Behavioral Changes and Oxidative Stress in an Animal Model of Mania.Mol Neurobiol. 2019 Apr;56(4):2379-2393. doi: 10.1007/s12035-018-1226-2. Epub 2018 Jul 20. Mol Neurobiol. 2019. PMID: 30027342
References
-
- Yao R, Cooper G M. Science. 1995;267:2003–2006. - PubMed
-
- Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Science. 1997;275:661–665. - PubMed
-
- Franke T F, Yang S-I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. Cell. 1995;81:727–736. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

