A tool for functional plant genomics: chimeric RNA/DNA oligonucleotides cause in vivo gene-specific mutations
- PMID: 10411951
- PMCID: PMC17592
- DOI: 10.1073/pnas.96.15.8774
A tool for functional plant genomics: chimeric RNA/DNA oligonucleotides cause in vivo gene-specific mutations
Abstract
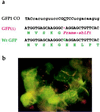
Self-complementary chimeric oligonucleotides (COs) composed of DNA and modified RNA residues were evaluated as a means to (i) create stable, site-specific base substitutions in a nuclear gene and (ii) introduce a frameshift in a nuclear transgene in plant cells. To demonstrate the creation of allele-specific mutations in a member of a gene family, COs were designed to target the codon for Pro-196 of SuRA, a tobacco acetolactate synthase (ALS) gene. An amino acid substitution at Pro-196 of ALS confers a herbicide-resistance phenotype that can be used as a selectable marker in plant cells. COs were designed to contain a 25-nt homology domain comprised of a five-deoxyribonucleotide region (harboring a single base mismatch to the native ALS sequence) flanked by regions each composed of 10 ribonucleotides. After recovery of herbicide-resistant tobacco cells on selective medium, DNA sequence analyses identified base conversions in the ALS gene at the codon for Pro-196. To demonstrate a site-specific insertion of a single base into a targeted gene, COs were used to restore expression of an inactive green fluorescent protein transgene that had been designed to contain a single base deletion. Recovery of fluorescent cells confirmed the deletion correction. Our results demonstrate the application of a technology to modify individual genetic loci by catalyzing either a base substitution or a base addition to specific nuclear genes; this approach should have great utility in the area of plant functional genomics.
Figures




Comment in
-
Gene therapy in plants.Proc Natl Acad Sci U S A. 1999 Jul 20;96(15):8321-3. doi: 10.1073/pnas.96.15.8321. Proc Natl Acad Sci U S A. 1999. PMID: 10411868 Free PMC article. No abstract available.
Similar articles
-
Chimeric RNA/DNA oligonucleotide-based site-specific modification of the tobacco acetolactate syntase gene.Plant Physiol. 2003 May;132(1):174-84. doi: 10.1104/pp.102.016857. Plant Physiol. 2003. PMID: 12746523 Free PMC article.
-
Chimeric RNA/DNA oligonucleotide-directed gene targeting in rice.Plant Cell Rep. 2004 Feb;22(7):509-12. doi: 10.1007/s00299-003-0698-2. Epub 2003 Nov 21. Plant Cell Rep. 2004. PMID: 14634786
-
Homologous recombination in plant cells after Agrobacterium-mediated transformation.Plant Cell. 1990 May;2(5):415-25. doi: 10.1105/tpc.2.5.415. Plant Cell. 1990. PMID: 2152167 Free PMC article.
-
Amino acids conferring herbicide resistance in tobacco acetohydroxyacid synthase.GM Crops. 2010 Mar-Apr;1(2):62-7. doi: 10.4161/gmcr.1.2.10856. GM Crops. 2010. PMID: 21865873 Review.
-
Gene repair and mutagenesis mediated by chimeric RNA-DNA oligonucleotides: chimeraplasty for gene therapy and conversion of single nucleotide polymorphisms (SNPs).Biochim Biophys Acta. 2002 May 21;1587(1):1-6. doi: 10.1016/s0925-4439(02)00068-6. Biochim Biophys Acta. 2002. PMID: 12009417 Review.
Cited by
-
Genome Editing and Protein Energy Malnutrition.Adv Exp Med Biol. 2023;1396:215-232. doi: 10.1007/978-981-19-5642-3_15. Adv Exp Med Biol. 2023. PMID: 36454470
-
Biotechnological Advances to Improve Abiotic Stress Tolerance in Crops.Int J Mol Sci. 2022 Oct 10;23(19):12053. doi: 10.3390/ijms231912053. Int J Mol Sci. 2022. PMID: 36233352 Free PMC article. Review.
-
Rescue of dystrophin expression in mdx mouse muscle by RNA/DNA oligonucleotides.Proc Natl Acad Sci U S A. 2000 May 9;97(10):5363-8. doi: 10.1073/pnas.97.10.5363. Proc Natl Acad Sci U S A. 2000. PMID: 10805797 Free PMC article.
-
Oligonucleotide-directed mutagenesis for precision gene editing.Plant Biotechnol J. 2016 Feb;14(2):496-502. doi: 10.1111/pbi.12496. Epub 2015 Oct 27. Plant Biotechnol J. 2016. PMID: 26503400 Free PMC article. Review.
-
Targeted gene repair -- in the arena.J Clin Invest. 2003 Sep;112(5):632-6. doi: 10.1172/JCI19777. J Clin Invest. 2003. PMID: 12952907 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

