Ultrastructural localization of the alpha4-subunit of the neuronal acetylcholine nicotinic receptor in the rat substantia nigra
- PMID: 10414976
- PMCID: PMC6782828
- DOI: 10.1523/JNEUROSCI.19-15-06475.1999
Ultrastructural localization of the alpha4-subunit of the neuronal acetylcholine nicotinic receptor in the rat substantia nigra
Abstract
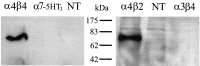
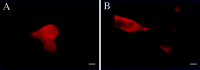
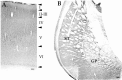
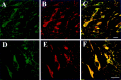
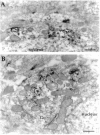
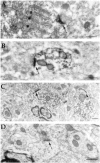
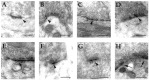
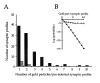
The distribution of the alpha4-subunit of the neuronal nicotinic acetylcholine receptor (nAChR) in the rat brain was examined at light and electron microscopy levels using immunohistochemical staining. In the present study we demonstrate the specificity, in both tissue homogenates and brain sections, of a polyclonal antibody raised against the rat nAChR alpha4-subunit. The characterization of this antibody involved: (1) Western blot analysis of rat brain homogenates and membrane extracts from cells previously transfected with diverse combinations of neuronal nAChR subunits, and (2) immunohistochemistry using transfected cells and rat brain tissue. At the light microscope level, the alpha4-subunit-like-immunoreactivity (LI) was widely distributed in the rat brain and matched the distribution of the alpha4-subunit transcripts observed previously by in situ hybridization. Strong immunohistochemical labeling was detected in the mesencephalic dopaminergic nuclei. The nAChRs in this region are thought to be responsible for the modulation of dopaminergic transmission. The neurotransmitter identity of alpha4-immunolabeled neurons in the substantia nigra pars compacta (SNpc) and the ventral tegmental area was thus assessed by investigating the possible colocalization of the nAChR alpha4-subunit with tyrosine hydroxylase using confocal microscopy. The double labeling experiments unambiguously indicated that the alpha4-subunit-LI is present in dopaminergic neurons. At the electron microscope level, the neurons in the SNpc exhibited alpha4-subunit-LI in association with a minority of postsynaptic densities, suggesting that the alpha4-subunit may be a component of functional nAChRs mediating synaptic transmission between midbrain cholinergic neurons and mesencephalic dopaminergic neurons.
Figures


References
-
- Beninato M, Spencer RF. A cholinergic projection to the rat substantia nigra from the pedunculopontine tegmental nucleus. Brain Res. 1987;412:169–174. - PubMed
-
- Beninato M, Spencer RF. The cholinergic innervation of the rat substantia nigra: a light and electron microscopic immunohistochemical study. Exp Brain Res. 1988;72:178–184. - PubMed
-
- Bertrand D, Changeux JP. Nicotinic receptor: an allosteric protein specialized for intercellular communication. Semin Neurosci. 1995;7:75–90.
-
- Blaha C, Allen L, Das S, Inglis W, Latimer M, Vincent S, Winn P. Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. J Neurosci. 1996;16:714–722. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
