Smell and taste perception in Drosophila melanogaster larva: toxin expression studies in chemosensory neurons
- PMID: 10414987
- PMCID: PMC6782832
- DOI: 10.1523/JNEUROSCI.19-15-06599.1999
Smell and taste perception in Drosophila melanogaster larva: toxin expression studies in chemosensory neurons
Abstract
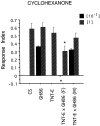
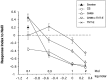
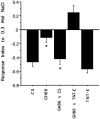
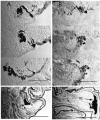
GAL4-driven targeted expression of tetanus toxin light chain (UAS-TeTxLC) in a subset of chemosensory neurons of the larval antennomaxillary complex (AMC) and pharynx causes abnormal chemosensory behavior in Drosophila melanogaster. Consistent with strongest staining in the dorsal organ (DO), the presumed olfactory organ of the AMC, tetanus toxin-expressing larvae subjected to an olfactory preference assay show anosmic behavior to most volatile substances tested. Furthermore, we observed reduced responses to sodium chloride, fructose, and sucrose in gustatory plate assays. Surprisingly, the entire subset of labeled sensory neurons from the terminal (maxillary) organ (TO) of the AMC was found to project via the antennal nerve to the larval antennal lobe region. The maxillary nerve remained completely unstained. Hence, a subset of neurons from the TO builds an anatomical entity with projections from the DO. Our results suggest that the AMC contains both olfactory and gustatory sensilla, and that the DO is the main olfactory organ in larvae.
Figures





Similar articles
-
The organization of the chemosensory system in Drosophila melanogaster: a review.Cell Tissue Res. 1994 Jan;275(1):3-26. doi: 10.1007/BF00305372. Cell Tissue Res. 1994. PMID: 8118845 Review.
-
Adult-like complexity of the larval antennal lobe of D. melanogaster despite markedly low numbers of odorant receptor neurons.J Comp Neurol. 2002 Apr 15;445(4):374-87. doi: 10.1002/cne.10188. J Comp Neurol. 2002. PMID: 11920714
-
Behavioral Analysis of Bitter Taste Perception in Drosophila Larvae.Chem Senses. 2016 Jan;41(1):85-94. doi: 10.1093/chemse/bjv061. Epub 2015 Oct 28. Chem Senses. 2016. PMID: 26512069
-
Larval chemosensory projections and invasion of adult afferents in the antennal lobe of Drosophila.J Neurobiol. 1997 Mar;32(3):281-97. doi: 10.1002/(sici)1097-4695(199703)32:3<281::aid-neu3>3.0.co;2-3. J Neurobiol. 1997. PMID: 9058321
-
A behavior-based circuit model of how outcome expectations organize learned behavior in larval Drosophila.Learn Mem. 2011 Sep 26;18(10):639-53. doi: 10.1101/lm.2163411. Print 2011 Oct. Learn Mem. 2011. PMID: 21946956 Review.
Cited by
-
Embryonic origin of olfactory circuitry in Drosophila: contact and activity-mediated interactions pattern connectivity in the antennal lobe.PLoS Biol. 2012;10(10):e1001400. doi: 10.1371/journal.pbio.1001400. Epub 2012 Oct 2. PLoS Biol. 2012. PMID: 23055825 Free PMC article.
-
The extracellular matrix protein artichoke is required for integrity of ciliated mechanosensory and chemosensory organs in Drosophila embryos.Genetics. 2014 Apr;196(4):1091-102. doi: 10.1534/genetics.113.156323. Epub 2014 Feb 4. Genetics. 2014. PMID: 24496014 Free PMC article.
-
Shedding light on toxicity of SARS-CoV-2 peptides in aquatic biota: A study involving neotropical mosquito larvae (Diptera: Culicidae).Environ Pollut. 2021 Nov 15;289:117818. doi: 10.1016/j.envpol.2021.117818. Epub 2021 Jul 20. Environ Pollut. 2021. PMID: 34333265 Free PMC article.
-
Active Mechanisms of Vibration Encoding and Frequency Filtering in Central Mechanosensory Neurons.Neuron. 2017 Oct 11;96(2):446-460.e9. doi: 10.1016/j.neuron.2017.09.004. Epub 2017 Sep 21. Neuron. 2017. PMID: 28943231 Free PMC article.
-
The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae.Proc Natl Acad Sci U S A. 2008 Apr 29;105(17):6433-8. doi: 10.1073/pnas.0801007105. Epub 2008 Apr 21. Proc Natl Acad Sci U S A. 2008. PMID: 18427108 Free PMC article.
References
-
- Aceves-Piña EO, Quinn WG. Learning in normal and mutant Drosophila larvae. Science. 1979;206:93–95. - PubMed
-
- Altner H, Prillinger L. Ultrastructure of invertebrate chemo-, thermo-, and hygroreceptors and its functional significance. Int Rev Cytol. 1980;67:69–131.
-
- Altner H, Sass H, Altner I. Relationship between structure and function of antennal chemo-, hygro-, and thermoreceptive sensilla in Periplaneta americana. Cell Tissue Res. 1977;176:389–405. - PubMed
-
- Ashburner M. Drosophila. A laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989.
-
- Ayyub C, Paranjape J, Rodriques V, Siddiqi O. Genetics of the olfactory behaviour in Drosophila melanogaster. J Neurogenet. 1990;6:285–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
