Coordination of fast and slow rhythmic neuronal circuits
- PMID: 10414994
- PMCID: PMC6782802
- DOI: 10.1523/JNEUROSCI.19-15-06650.1999
Coordination of fast and slow rhythmic neuronal circuits
Abstract
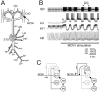
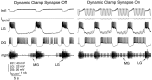
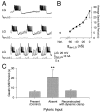
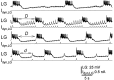
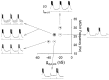
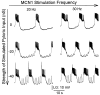
Interactions among rhythmically active neuronal circuits that oscillate at different frequencies are important for generating complex behaviors, yet little is known about the underlying cellular mechanisms. We addressed this issue in the crab stomatogastric ganglion (STG), which contains two distinct but interacting circuits. These circuits generate the gastric mill rhythm (cycle period, approximately 10 sec) and the pyloric rhythm (cycle period, approximately 1 sec). When the identified modulatory projection neuron named modulatory commissural neuron 1 (MCN1) is activated, the gastric mill motor pattern is generated by interactions among MCN1 and two STG neurons [the lateral gastric (LG) neuron and interneuron 1]. We show that, during MCN1 stimulation, an identified synapse from the pyloric circuit onto the gastric mill circuit is pivotal for determining the gastric mill cycle period and the gastric-pyloric rhythm coordination. To examine the role of this intercircuit synapse, we replaced it with a computational equivalent via the dynamic-clamp technique. This enabled us to manipulate better the timing and strength of this synapse. We found this synapse to be necessary for production of the normal gastric mill cycle period. The synapse acts, during each LG neuron interburst, to boost rhythmically the influence of the modulatory input from MCN1 to LG and thereby to hasten LG neuron burst onset. The two rhythms become coordinated because LG burst onset occurs with a constant latency after the onset of the triggering pyloric input. These results indicate that intercircuit synapses can enable an oscillatory circuit to control the speed of a slower oscillatory circuit, as well as provide a mechanism for intercircuit coordination.
Figures




References
-
- Bartos M, Nusbaum MP. Frequency regulation of a slow rhythm by periodic inputs from a fast rhythm in the crab stomatogastric ganglion. Soc Neurosci Abstr. 1997b;23:476.
-
- Cattaert D, Clarac F. Influence of walking on swimmeret beating in the lobster Homarus gammarus. J Neurobiol. 1983;14:421–439. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
