Monoamine control of the pacemaker kernel and cycle frequency in the lobster pyloric network
- PMID: 10415000
- PMCID: PMC6782821
- DOI: 10.1523/JNEUROSCI.19-15-06712.1999
Monoamine control of the pacemaker kernel and cycle frequency in the lobster pyloric network
Abstract
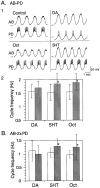
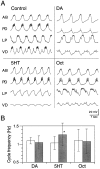
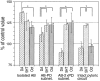
The monoamines dopamine (DA), serotonin (5HT), and octopamine (Oct) can each sculpt a unique motor pattern from the pyloric network in the stomatogastric ganglion (STG) of the spiny lobster Panulirus interruptus. In this paper we investigate the contribution of individual network components in determining the specific amine-induced cycle frequency. We used photoinactivation of identified neurons and pharmacological blockade of synapses to isolate the anterior burster (AB) and pyloric dilator (PD) neurons. Bath application of DA, 5HT, or Oct enhanced cycle frequency in an isolated AB neuron, with DA generating the most rapid oscillations and Oct the slowest. When an AB-PD or AB-2xPD subnetworks were tested, DA often reduced the ongoing cycle frequency, whereas 5HT and Oct both evoked similar accelerations in cycle frequency. However, in the intact pyloric network, both DA and Oct either reduced or did not alter the cycle frequency, whereas 5HT continued to enhance the cycle frequency as before. Our results show that the major target of 5HT in altering the pyloric cycle frequency is the AB neuron, whereas DA's effects on the AB-2xPD subnetwork are critical in understanding its modulation of the cycle frequency. Octopamine's effects on cycle frequency require an understanding of its modulation of the feedback inhibition to the AB-PD group from the lateral pyloric neuron, which constrains the pacemaker group to oscillate more slowly than it would alone. We have thus demonstrated that the relative importance of the different network components in determining the final cycle frequency is not fixed but can vary under different modulatory conditions.
Figures






References
-
- Abbott LF, Marder E, Hooper SL. Oscillating networks: control of burst duration by electrically coupled neurons. Neural Comput. 1991;3:487–497. - PubMed
-
- Ayali A, Harris-Warrick RM. Combined effects of amine modulation and CPG network interactions in the lobster stomatogastric ganglion. Brain Res. 1998;794:155–161. - PubMed
-
- Ayali A, Johnson BR, Harris-Warrick RM. Dopamine modulates graded and spike-evoked synaptic inhibition independently at single synapses in pyloric network of lobster. J Neurophysiol. 1998;79:2063–2069. - PubMed
-
- Baro DJ, Levini RM, Kim MT, Willms AR, Cole CL, Rodriguez HE, Harris-Warrick RM. Quantitative single-cell–reverse transcription-PCR demonstrates that A-current magnitude varies as a linear function of shal gene expression in identified stomatogastric neurons. J Neurosci. 1997;17:6597–6610. - PMC - PubMed
-
- Bidaut M. Pharmacological dissection of pyloric network of the lobster stomatogastric ganglion using picrotoxin. J Neurophysiol. 1980;44:1089–1101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
