Citrobacter freundii invades and replicates in human brain microvascular endothelial cells
- PMID: 10417193
- PMCID: PMC96726
- DOI: 10.1128/IAI.67.8.4208-4215.1999
Citrobacter freundii invades and replicates in human brain microvascular endothelial cells
Abstract
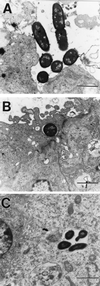
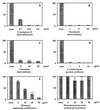
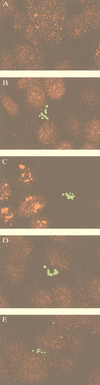
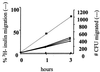
Neonatal bacterial meningitis remains a disease with unacceptable rates of morbidity and mortality despite the availability of effective antimicrobial therapy. Citrobacter spp. cause neonatal meningitis but are unique in their frequent association with brain abscess formation. The pathogenesis of Citrobacter spp. causing meningitis and brain abscess is not well characterized; however, as with other meningitis-causing bacteria (e.g., Escherichia coli K1 and group B streptococci), penetration of the blood-brain barrier must occur. In an effort to understand the pathogenesis of Citrobacter spp. causing meningitis, we have used the in vitro blood-brain barrier model of human brain microvascular endothelial cells (HBMEC) to study the interaction between C. freundii and HBMEC. In this study, we show that C. freundii is capable of invading and trancytosing HBMEC in vitro. Invasion of HBMEC by C. freundii was determined to be dependent on microfilaments, microtubules, endosome acidification, and de novo protein synthesis. Immunofluorescence microscopy studies revealed that microtubules aggregated after HBMEC came in contact with C. freundii; furthermore, the microtubule aggregation was time dependent and seen with C. freundii but not with noninvasive E. coli HB101 and meningitic E. coli K1. Also in contrast to other meningitis-causing bacteria, C. freundii is able to replicate within HBMEC. This is the first demonstration of a meningitis-causing bacterium capable of intracellular replication within BMEC. The important determinants of the pathogenesis of C. freundii causing meningitis and brain abscess may relate to invasion of and intracellular replication in HBMEC.
Figures
References
-
- Cundell D R, Gerard N P, Gerard C, Idanpaan-Heikkila I, Tuomanen E I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. - PubMed
-
- Davies P J, Davies D R, Levitzki A, Maxfield F R, Milhaud P, Willingham M C, Pastan I H. Transglutaminase is essential in receptor-mediated endocytosis of alpha 2-macroglobulin and polypeptide hormones. Nature. 1980;283:162–167. - PubMed
-
- Donnenberg M S, Donohue-Rolfe A, Keusch G T. A comparison of HEp-2 cell invasion by enteropathogenic and enteroinvasive Escherichia coli. FEMS Microbiol Lett. 1990;57:83–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

