Mutational analysis and membrane topology of ComP, a quorum-sensing histidine kinase of Bacillus subtilis controlling competence development
- PMID: 10419951
- PMCID: PMC103584
- DOI: 10.1128/JB.181.15.4540-4548.1999
Mutational analysis and membrane topology of ComP, a quorum-sensing histidine kinase of Bacillus subtilis controlling competence development
Abstract
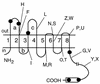
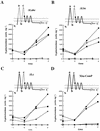
ComP is a sensor histidine kinase of Bacillus subtilis required for the signal transduction pathway that initiates the development of competence for genetic transformation. It is believed that ComP senses the presence of ComX, a modified extracellular peptide pheromone, and donates a phosphate to ComA, thereby activating this transcription factor for binding to the srfA promoter. In the present study, fusions to the Escherichia coli proteins PhoA and LacZ and analysis of its susceptibility to the protease kallikrein were used to probe the membrane topology of ComP. These data suggest that ComP contains six or eight membrane-spanning segments and two large extracytoplasmic loops in its N-terminal membrane-associated domain. Deletions were introduced involving the large extracellular loops to explore the role of the N-terminal domain of ComP in signal transduction. The absence of the second loop conferred a phenotype in which ComP was active in the absence of ComX. The implications of these data are discussed.
Figures




Similar articles
-
Specificity and genetic polymorphism of the Bacillus competence quorum-sensing system.J Bacteriol. 2001 Jan;183(2):451-60. doi: 10.1128/JB.183.2.451-460.2001. J Bacteriol. 2001. PMID: 11133937 Free PMC article.
-
The primary role of comA in establishment of the competent state in Bacillus subtilis is to activate expression of srfA.J Bacteriol. 1991 Nov;173(22):7269-74. doi: 10.1128/jb.173.22.7269-7274.1991. J Bacteriol. 1991. PMID: 1938921 Free PMC article.
-
Conservation of genes and processes controlled by the quorum response in bacteria: characterization of genes controlled by the quorum-sensing transcription factor ComA in Bacillus subtilis.Mol Microbiol. 2005 Aug;57(4):1159-74. doi: 10.1111/j.1365-2958.2005.04749.x. Mol Microbiol. 2005. PMID: 16091051
-
Transcriptional regulation of Bacillus subtilis glucose starvation-inducible genes: control of gsiA by the ComP-ComA signal transduction system.J Bacteriol. 1992 Jul;174(13):4361-73. doi: 10.1128/jb.174.13.4361-4373.1992. J Bacteriol. 1992. PMID: 1378051 Free PMC article.
-
Anaerobic growth of a "strict aerobe" (Bacillus subtilis).Annu Rev Microbiol. 1998;52:165-90. doi: 10.1146/annurev.micro.52.1.165. Annu Rev Microbiol. 1998. PMID: 9891797 Review.
Cited by
-
Molecular genetics of surfactin and its effects on different sub-populations of Bacillus subtilis.Biotechnol Rep (Amst). 2021 Oct 26;32:e00686. doi: 10.1016/j.btre.2021.e00686. eCollection 2021 Dec. Biotechnol Rep (Amst). 2021. PMID: 34786355 Free PMC article. Review.
-
The H2O2 stress-responsive regulator PerR positively regulates srfA expression in Bacillus subtilis.J Bacteriol. 2005 Oct;187(19):6659-67. doi: 10.1128/JB.187.19.6659-6667.2005. J Bacteriol. 2005. PMID: 16166527 Free PMC article.
-
Draft genome comparison of representatives of the three dominant genotype groups of dairy Bacillus licheniformis strains.Appl Environ Microbiol. 2014 Jun;80(11):3453-62. doi: 10.1128/AEM.00065-14. Epub 2014 Mar 21. Appl Environ Microbiol. 2014. PMID: 24657871 Free PMC article.
-
Insights into the structure and function of the histidine kinase ComP from Bacillus amyloliquefaciens based on molecular modeling.Biosci Rep. 2022 Oct 28;42(10):BSR20220352. doi: 10.1042/BSR20220352. Biosci Rep. 2022. PMID: 36052710 Free PMC article.
-
Novel methods for genetic transformation of natural Bacillus subtilis isolates used to study the regulation of the mycosubtilin and surfactin synthetases.Appl Environ Microbiol. 2007 Jun;73(11):3490-6. doi: 10.1128/AEM.02751-06. Epub 2007 Apr 6. Appl Environ Microbiol. 2007. PMID: 17416694 Free PMC article.
References
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidam J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates/Wiley Interscience; 1994.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

