MgATP binding and hydrolysis determinants of NtrC, a bacterial enhancer-binding protein
- PMID: 10419963
- PMCID: PMC103596
- DOI: 10.1128/JB.181.15.4628-4638.1999
MgATP binding and hydrolysis determinants of NtrC, a bacterial enhancer-binding protein
Abstract
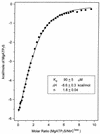
When phosphorylated, the dimeric form of nitrogen regulatory protein C (NtrC) of Salmonella typhimurium forms a larger oligomer(s) that can hydrolyze ATP and hence activate transcription by the sigma(54)-holoenzyme form of RNA polymerase. Studies of Mg-nucleoside triphosphate binding using a filter-binding assay indicated that phosphorylation is not required for nucleotide binding but probably controls nucleotide hydrolysis per se. Studies of binding by isothermal titration calorimetry indicated that the apparent K(d) of unphosphorylated NtrC for MgATPgammaS is 100 microM at 25 degrees C, and studies by filter binding indicated that the concentration of MgATP required for half-maximal binding is 130 microM at 37 degrees C. Filter-binding studies with mutant forms of NtrC defective in ATP hydrolysis implicated two regions of its central domain directly in nucleotide binding and three additional regions in hydrolysis. All five are highly conserved among activators of sigma(54)-holoenzyme. Regions implicated in binding are the Walker A motif and the region around residues G355 to R358, which may interact with the nucleotide base. Regions implicated in nucleotide hydrolysis are residues S207 and E208, which have been proposed to lie in a region analogous to the switch I effector region of p21(ras) and other purine nucleotide-binding proteins; residue R294, which may be a catalytic residue; and residue D239, which is the conserved aspartate in the putative Walker B motif. D239 appears to play a role in binding the divalent cation essential for nucleotide hydrolysis. Electron paramagnetic resonance analysis of Mn(2+) binding indicated that the central domain of NtrC does not bind divalent cation strongly in the absence of nucleotide.
Figures



Similar articles
-
Repressor forms of the enhancer-binding protein NrtC: some fail in coupling ATP hydrolysis to open complex formation by sigma 54-holoenzyme.J Mol Biol. 1996 Jul 19;260(3):317-31. doi: 10.1006/jmbi.1996.0403. J Mol Biol. 1996. PMID: 8757796
-
Constitutive forms of the enhancer-binding protein NtrC: evidence that essential oligomerization determinants lie in the central activation domain.J Mol Biol. 1995 Jun 16;249(4):700-13. doi: 10.1006/jmbi.1995.0330. J Mol Biol. 1995. PMID: 7602583
-
Glutamate at the site of phosphorylation of nitrogen-regulatory protein NTRC mimics aspartyl-phosphate and activates the protein.J Mol Biol. 1993 Jul 5;232(1):67-78. doi: 10.1006/jmbi.1993.1370. J Mol Biol. 1993. PMID: 8331671
-
Structures and organisation of AAA+ enhancer binding proteins in transcriptional activation.J Struct Biol. 2006 Oct;156(1):190-9. doi: 10.1016/j.jsb.2006.01.006. Epub 2006 Feb 20. J Struct Biol. 2006. PMID: 16531068 Review.
-
The bacterial enhancer-binding protein NtrC as a molecular machine.Cold Spring Harb Symp Quant Biol. 1998;63:157-66. doi: 10.1101/sqb.1998.63.157. Cold Spring Harb Symp Quant Biol. 1998. PMID: 10384279 Review. No abstract available.
Cited by
-
ATP ground- and transition states of bacterial enhancer binding AAA+ ATPases support complex formation with their target protein, sigma54.Structure. 2007 Apr;15(4):429-40. doi: 10.1016/j.str.2007.02.007. Structure. 2007. PMID: 17437715 Free PMC article.
-
Functional roles of the pre-sensor I insertion sequence in an AAA+ bacterial enhancer binding protein.Mol Microbiol. 2009 Aug;73(4):519-33. doi: 10.1111/j.1365-2958.2009.06744.x. Epub 2009 May 25. Mol Microbiol. 2009. PMID: 19486295 Free PMC article.
-
The role of the conserved phenylalanine in the sigma54-interacting GAFTGA motif of bacterial enhancer binding proteins.Nucleic Acids Res. 2009 Oct;37(18):5981-92. doi: 10.1093/nar/gkp658. Epub 2009 Aug 19. Nucleic Acids Res. 2009. PMID: 19692583 Free PMC article.
-
Sigma54-dependent transcription activator phage shock protein F of Escherichia coli: a fragmentation approach to identify sequences that contribute to self-association.Biochem J. 2004 Mar 15;378(Pt 3):735-44. doi: 10.1042/BJ20031464. Biochem J. 2004. PMID: 14659000 Free PMC article.
-
Phosphorylation-induced signal propagation in the response regulator ntrC.J Bacteriol. 2000 Sep;182(18):5188-95. doi: 10.1128/JB.182.18.5188-5195.2000. J Bacteriol. 2000. PMID: 10960104 Free PMC article.
References
-
- Abrahams J P, Leslie A G, Lutter R, Walker J E. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–628. - PubMed
-
- Al-Shawi M K, Pasonage D, Senior A E. Directed mutagenesis of the strongly conserved aspartate 242 in the β-subunit of Escherichia coli proton-ATPase. J Biol Chem. 1988;263:19633–19639. - PubMed
-
- Ausubel F A, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994.
-
- Berchtold H, Reshetnikova L, Reiser C O, Schirmer N K, Sprinzl M, Hilgenfeld R. Crystal structure of active elongation factor Tu reveals major domain rearrangements. Nature. 1993;365:126–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

