Alteration of the repressor activity of MarR, the negative regulator of the Escherichia coli marRAB locus, by multiple chemicals in vitro
- PMID: 10419969
- PMCID: PMC103602
- DOI: 10.1128/JB.181.15.4669-4672.1999
Alteration of the repressor activity of MarR, the negative regulator of the Escherichia coli marRAB locus, by multiple chemicals in vitro
Abstract
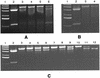
MarR negatively regulates expression of the multiple antibiotic resistance operon (marRAB) in Escherichia coli. In this study, it was demonstrated that sodium salicylate, plumbagin, 2, 4-dinitrophenol, and menadione-inducers of the marRAB operon in whole cells-all interfered with the repressor activity of MarR in vitro. It is proposed that these compounds can interact directly with MarR to affect its repressor activity.
Figures

References
-
- Ahmed M, Borsch C M, Taylor S S, Vazquez-Laslop N, Neyfakh A A. A protein that activates expression of a multidrug efflux transporter upon binding the transporter substrates. J Biol Chem. 1994;269:28506–28513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

