Single-channel properties of BK-type calcium-activated potassium channels at a cholinergic presynaptic nerve terminal
- PMID: 10420003
- PMCID: PMC2269469
- DOI: 10.1111/j.1469-7793.1999.0639p.x
Single-channel properties of BK-type calcium-activated potassium channels at a cholinergic presynaptic nerve terminal
Abstract
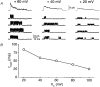
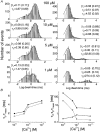
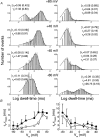
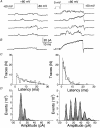
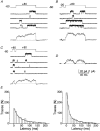
1. A high-conductance calcium-activated potassium channel (BK KCa) was characterized at a cholinergic presynaptic nerve terminal using the calyx synapse isolated from the chick ciliary ganglion. 2. The channel had a conductance of 210 pS in a 150 mM:150 mM K+ gradient, was highly selective for K+ over Na+, and was sensitive to block by external charybdotoxin or tetraethylammonium (TEA) and by internal Ba2+. At +60 mV it was activated by cytoplasmic calcium [Ca2+]i with a Kd of approximately 0.5 microM and a Hill coefficient of approximately 2.0. At 10 microM [Ca2+]i the channel was 50 % activated (V) at -8.0 mV with a voltage dependence (Boltzmann slope-factor) of 32.7 mV. The V values hyperpolarized with an increase in [Ca2+]i while the slope factors decreased. There were no overt differences in conductance or [Ca2+]i sensitivity between BK channels from the transmitter release face and the non-release face. 3. Open and closed times were fitted by two and three exponentials, respectively. The slow time constants were strongly affected by both [Ca2+]i and membrane potential changes. 4. In cell-attached patch recordings BK channel opening was enhanced by a prepulse permissive for calcium influx through the patch, suggesting that the channel can be activated by calcium ion influx through neighbouring calcium channels. 5. The properties of the presynaptic BK channel are well suited for rapid activation during the presynaptic depolarization and Ca2+ influx that are associated with transmitter release. This channel may play an important role in terminating release by rapid repolarization of the action potential.
Figures



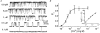
Similar articles
-
Large-conductance calcium-activated potassium channels in neonatal rat intracardiac ganglion neurons.Pflugers Arch. 2001 Feb;441(5):629-38. doi: 10.1007/s004240000471. Pflugers Arch. 2001. PMID: 11294244
-
Large-conductance calcium-activated potassium channels of cultured rat melanotrophs.J Membr Biol. 1996 Apr;150(3):219-30. doi: 10.1007/s002329900046. J Membr Biol. 1996. PMID: 8661991
-
Voltage-gated potassium channels activated during action potentials in layer V neocortical pyramidal neurons.J Neurophysiol. 2000 Jan;83(1):70-80. doi: 10.1152/jn.2000.83.1.70. J Neurophysiol. 2000. PMID: 10634854
-
The calyx-type synapse of the chick ciliary ganglion as a model of fast cholinergic transmission.Can J Physiol Pharmacol. 1992;70 Suppl:S73-7. doi: 10.1139/y92-246. Can J Physiol Pharmacol. 1992. PMID: 1338300 Review.
-
Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage.Biol Res. 2006;39(3):385-401. doi: 10.4067/s0716-97602006000300003. Epub 2006 Nov 7. Biol Res. 2006. PMID: 17106573 Review.
Cited by
-
Regulation of Neurotransmitter Release by K+ Channels.Adv Neurobiol. 2023;33:305-331. doi: 10.1007/978-3-031-34229-5_12. Adv Neurobiol. 2023. PMID: 37615872
-
Large conductance voltage-and calcium-activated K+ (BK) channel in health and disease.Front Pharmacol. 2024 Mar 22;15:1373507. doi: 10.3389/fphar.2024.1373507. eCollection 2024. Front Pharmacol. 2024. PMID: 38584598 Free PMC article. Review.
-
Opening large-conductance potassium channels selectively induced cell death of triple-negative breast cancer.BMC Cancer. 2020 Jun 26;20(1):595. doi: 10.1186/s12885-020-07071-1. BMC Cancer. 2020. PMID: 32586284 Free PMC article.
-
Atomistic Simulation of Voltage Activation of a Truncated BK Channel.bioRxiv [Preprint]. 2025 Jun 14:2025.01.08.631907. doi: 10.1101/2025.01.08.631907. bioRxiv. 2025. PMID: 40661448 Free PMC article. Preprint.
-
Contribution of BK channels to action potential repolarisation at minimal cytosolic Ca2+ concentration in chromaffin cells.Pflugers Arch. 2011 Oct;462(4):545-57. doi: 10.1007/s00424-011-0991-9. Epub 2011 Jul 14. Pflugers Arch. 2011. PMID: 21755285
References
-
- Astrand P, Stjarne L. A calcium-dependent component of the action potential in sympathetic nerve terminals in rat tail artery. Pflügers Archiv. 1991;418:102–108. - PubMed
-
- Bielefeldt K, Jackson MB. A calcium-activated potassium channel causes frequency-dependent action-potential failures in a mammalian nerve terminal. Journal of Neurophysiology. 1993;70:284–298. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

