Transcription elongation factor hSPT5 stimulates mRNA capping
- PMID: 10421630
- PMCID: PMC316881
- DOI: 10.1101/gad.13.14.1774
Transcription elongation factor hSPT5 stimulates mRNA capping
Abstract
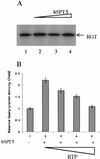
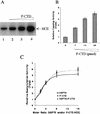
RNA polymerase II nascent transcripts are capped during pausing before elongation. Here we report that hSPT5, the human homolog of yeast elongation factor SPT5, interacts directly with the capping enzyme. hSPT5 stimulated capping enzyme guanylylation and mRNA capping by severalfold. Although RNA 5'-triphosphatase activity was unaffected, binding to this domain in the full-length enzyme is likely involved in the stimulation, as hSPT5 did not increase the activity of the guanylyltransferase fragment. Consistent with capping enzyme binding, TFIIH-phosphorylated CTD stimulated guanylylation, and this increase was not additive with hSPT5.
Figures


Similar articles
-
Interactions between fission yeast mRNA capping enzymes and elongation factor Spt5.J Biol Chem. 2002 May 31;277(22):19639-48. doi: 10.1074/jbc.M200015200. Epub 2002 Mar 13. J Biol Chem. 2002. PMID: 11893740
-
The Tat/TAR-dependent phosphorylation of RNA polymerase II C-terminal domain stimulates cotranscriptional capping of HIV-1 mRNA.Proc Natl Acad Sci U S A. 2003 Oct 28;100(22):12666-71. doi: 10.1073/pnas.1835726100. Epub 2003 Oct 20. Proc Natl Acad Sci U S A. 2003. PMID: 14569024 Free PMC article.
-
Separable functions of the fission yeast Spt5 carboxyl-terminal domain (CTD) in capping enzyme binding and transcription elongation overlap with those of the RNA polymerase II CTD.Mol Cell Biol. 2010 May;30(10):2353-64. doi: 10.1128/MCB.00116-10. Epub 2010 Mar 15. Mol Cell Biol. 2010. PMID: 20231361 Free PMC article.
-
Enzymology of RNA cap synthesis.Wiley Interdiscip Rev RNA. 2010 Jul-Aug;1(1):152-72. doi: 10.1002/wrna.19. Epub 2010 May 25. Wiley Interdiscip Rev RNA. 2010. PMID: 21956912 Free PMC article. Review.
-
Structure, mechanism, and evolution of the mRNA capping apparatus.Prog Nucleic Acid Res Mol Biol. 2001;66:1-40. doi: 10.1016/s0079-6603(00)66025-7. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11051760 Review. No abstract available.
Cited by
-
RNA polymerase II transcription elongation control.Chem Rev. 2013 Nov 13;113(11):8583-603. doi: 10.1021/cr400105n. Epub 2013 Aug 6. Chem Rev. 2013. PMID: 23919563 Free PMC article. Review. No abstract available.
-
Modulating HIV-1 replication by RNA interference directed against human transcription elongation factor SPT5.Retrovirology. 2004 Dec 27;1:46. doi: 10.1186/1742-4690-1-46. Retrovirology. 2004. PMID: 15620346 Free PMC article.
-
CDK9 keeps RNA polymerase II on track.Cell Mol Life Sci. 2021 Jul;78(14):5543-5567. doi: 10.1007/s00018-021-03878-8. Epub 2021 Jun 19. Cell Mol Life Sci. 2021. PMID: 34146121 Free PMC article. Review.
-
Involvement of Human Cellular Proteins and Structures in Realization of the HIV Life Cycle: A Comprehensive Review, 2024.Viruses. 2024 Oct 29;16(11):1682. doi: 10.3390/v16111682. Viruses. 2024. PMID: 39599797 Free PMC article. Review.
-
The yeast transcription elongation factor Spt4/5 is a sequence-specific RNA binding protein.Protein Sci. 2016 Sep;25(9):1710-21. doi: 10.1002/pro.2976. Epub 2016 Jul 15. Protein Sci. 2016. PMID: 27376968 Free PMC article.
References
-
- Dahmus ME. Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J Biol Chem. 1996;271:19009–19012. - PubMed
-
- Furuichi Y, Shatkin AJ. Encyclopedia of life sciences. London, UK: Macmillan; 1999. Caps on eukaryotic mRNAs. . (In press.)
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
