Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits
- PMID: 10426311
- PMCID: PMC3382971
- DOI: 10.1038/11330
Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits
Abstract
Febrile (fever-induced) seizures affect 3-5% of infants and young children. Despite the high incidence of febrile seizures, their contribution to the development of epilepsy later in life has remained controversial. Combining a new rat model of complex febrile seizures and patch clamp techniques, we determined that hyperthermia-induced seizures in the immature rat cause a selective presynaptic increase in inhibitory synaptic transmission in the hippocampus that lasts into adulthood. The long-lasting nature of these potent alterations in synaptic communication after febrile seizures does not support the prevalent view of the 'benign' nature of early-life febrile convulsions.
Figures
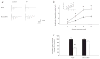


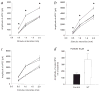
Comment in
-
Febrile convulsions: a 'benign' condition?Nat Med. 1999 Aug;5(8):871-2. doi: 10.1038/11308. Nat Med. 1999. PMID: 10426304 No abstract available.
References
-
- Shinnar S. In: Current Therapy in Neurological Disease. Johnson RT, editor. Decker; Philadelphia: 1990.
-
- Shinnar S. Prolonged febrile seizures and mesial temporal sclerosis. Ann Neurol. 1998;43:411–412. - PubMed
-
- Abou-Khalil B, Andermann E, Andermann F, Olivier A, Quesney LF. Temporal lobe epilepsy after prolonged febrile convulsions: excellent outcome after surgical treatment. Epilepsia. 1993;34:878–83. - PubMed
-
- Cendes F, et al. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures, and temporal lobe epilepsy: an MRI volumetric study. Neurology. 1993;43:1083–1087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

