Characterization of methanotrophic bacterial populations in soils showing atmospheric methane uptake
- PMID: 10427012
- PMCID: PMC91497
- DOI: 10.1128/AEM.65.8.3312-3318.1999
Characterization of methanotrophic bacterial populations in soils showing atmospheric methane uptake
Abstract
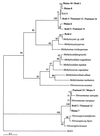
The global methane cycle includes both terrestrial and atmospheric processes and may contribute to feedback regulation of the climate. Most oxic soils are a net sink for methane, and these soils consume approximately 20 to 60 Tg of methane per year. The soil sink for atmospheric methane is microbially mediated and sensitive to disturbance. A decrease in the capacity of this sink may have contributed to the approximately 1%. year(-1) increase in the atmospheric methane level in this century. The organisms responsible for methane uptake by soils (the atmospheric methane sink) are not known, and factors that influence the activity of these organisms are poorly understood. In this study the soil methane-oxidizing population was characterized by both labelling soil microbiota with (14)CH(4) and analyzing a total soil monooxygenase gene library. Comparative analyses of [(14)C]phospholipid ester-linked fatty acid profiles performed with representative methane-oxidizing bacteria revealed that the soil sink for atmospheric methane consists of an unknown group of methanotrophic bacteria that exhibit some similarity to type II methanotrophs. An analysis of monooxygenase gene libraries from the same soil samples indicated that an unknown group of bacteria belonging to the alpha subclass of the class Proteobacteria was present; these organisms were only distantly related to extant methane-oxidizing strains. Studies on factors that affect the activity, population dynamics, and contribution to global methane flux of "atmospheric methane oxidizers" should be greatly facilitated by use of biomarkers identified in this study.
Figures



References
-
- Bender M, Conrad R. Kinetics of CH4 oxidation in oxic soils exposed to ambient air or high CH4 mixing ratios. FEMS Microbiol Ecol. 1992;101:261–270.
-
- Blake D R, Rowland F S. Continuing worldwide increase in tropospheric methane, 1978–1987. Science. 1988;239:1129–1131. - PubMed
-
- Bowman J P, Sly L I, Nicholls P D, Hayward A C. Revised taxonomy of the methanotrophs: description of Methylobacter gen. nov., emendation of Methylococcus, validation of Methylosinus and Methylocystis species, and a proposal that the family Methylococcaceae includes only the group I methanotrophs. Int J Syst Bacteriol. 1993;43:735–753.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

