Expression of the 180-kD ribosome receptor induces membrane proliferation and increased secretory activity in yeast
- PMID: 10427084
- PMCID: PMC3206573
- DOI: 10.1083/jcb.146.2.273
Expression of the 180-kD ribosome receptor induces membrane proliferation and increased secretory activity in yeast
Abstract
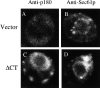
Expression of the canine 180-kD ribosome receptor (p180) in yeast cells resulted in a marked proliferation of intracellular membranes. The type of membranes observed varied with the expression of specific portions of p180. Rough membranes predominated when the ribosome binding domain of p180 was present, whereas expression constructs lacking this region resulted in smooth membranes. Northern analysis indicated that expression of the NH(2)-terminal 767 amino acids (DeltaCT), which include the ribosome binding domain, upregulated the transcription and translation of genes involved in exocytosis. The membranes that were proliferated were functional as these cells overcame a temperature-sensitive translocation defect. Most significantly, cells that overexpressed DeltaCT and proliferated rough endoplasmic reticulum exhibited severalfold higher levels of secretion of an ectopically expressed secretory protein. We conclude that p180 expression triggers a cascade of events leading to an increase in secretory potential akin to the terminal differentiation of mammalian secretory cells and tissues.
Figures










References
-
- Anderson R.G., Orci L., Brown M.S., Garcia S.L., Goldstein J.L. Ultrastructural analysis of crystalloid endoplasmic reticulum in UT-1 cells and its disappearance in response to cholesterol. J. Cell Sci. 1983;63:1–20. - PubMed
-
- Basson C.T., MacRae C.A., Schoenberg-Fejzo M., Morton C.C., Spinner N.B., Genin A., Krug E., Seidman J.G., Seidman C.E. Identification, characterization and chromosomal localization of the human homolog (hES) of ES/130. Genomics. 1996;35:628–631. - PubMed
-
- Borgese N., Mok W., Kreibich G., Sabatini D.D. Ribosomal-membrane interactionin vitro binding of ribosomes to microsomal membranes. J. Mol. Biol. 1974;88:559–580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

