N-Glycans mediate the apical sorting of a GPI-anchored, raft-associated protein in Madin-Darby canine kidney cells
- PMID: 10427087
- PMCID: PMC2156177
- DOI: 10.1083/jcb.146.2.313
N-Glycans mediate the apical sorting of a GPI-anchored, raft-associated protein in Madin-Darby canine kidney cells
Abstract
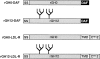
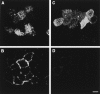
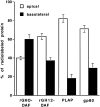
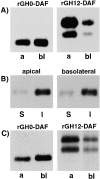
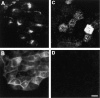
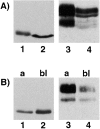
Glycosyl-phosphatidylinositol (GPI)- anchored proteins are preferentially transported to the apical cell surface of polarized Madin-Darby canine kidney (MDCK) cells. It has been assumed that the GPI anchor itself acts as an apical determinant by its interaction with sphingolipid-cholesterol rafts. We modified the rat growth hormone (rGH), an unglycosylated, unpolarized secreted protein, into a GPI-anchored protein and analyzed its surface delivery in polarized MDCK cells. The addition of a GPI anchor to rGH did not lead to an increase in apical delivery of the protein. However, addition of N-glycans to GPI-anchored rGH resulted in predominant apical delivery, suggesting that N-glycans act as apical sorting signals on GPI-anchored proteins as they do on transmembrane and secretory proteins. In contrast to the GPI-anchored rGH, a transmembrane form of rGH which was not raft-associated accumulated intracellularly. Addition of N-glycans to this chimeric protein prevented intracellular accumulation and led to apical delivery.
Figures

References
-
- Ahmed S.N., Brown D.A., London E. On the origin of sphingolipid-cholesterol rich detergent-insoluble domains in cell membranesphysiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 1997;36:10944–10953. - PubMed
-
- Alonso M.A., Fan L., Alarcon B. Multiple sorting signals determine apical localization of a non-glycosylated integral membrane protein. J. Biol. Chem. 1997;272:30748–30752. - PubMed
-
- Arreaza G., Brown D.A. Sorting and intracellular trafficking of a glycosylphosphatidylinositol-anchored protein and two hybrid transmembrane proteins with the same ectodomain in Madin-Darby canine kidney epithelial cells. J. Biol. Chem. 1995;270:23641–23647. - PubMed
-
- Brown D.A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. - PubMed
-
- Brown D.A., Rose J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

