ZASP: a new Z-band alternatively spliced PDZ-motif protein
- PMID: 10427098
- PMCID: PMC3206570
- DOI: 10.1083/jcb.146.2.465
ZASP: a new Z-band alternatively spliced PDZ-motif protein
Abstract
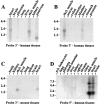
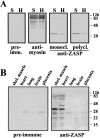
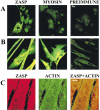
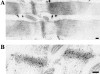
PDZ motifs are modular protein-protein interaction domains, consisting of 80-120 amino acid residues, whose function appears to be the direction of intracellular proteins to multiprotein complexes. In skeletal muscle, there are a few known PDZ-domain proteins, which include neuronal nitric oxide synthase and syntrophin, both of which are components of the dystrophin complex, and actinin-associated LIM protein, which binds to the spectrin-like repeats of alpha-actinin-2. Here, we report the identification and characterization of a new skeletal muscle protein containing a PDZ domain that binds to the COOH-terminal region of alpha-actinin-2. This novel 31-kD protein is specifically expressed in heart and skeletal muscle. Using antibodies produced to a fragment of the protein, we can show its location in the sarcomere at the level of the Z-band by immunoelectron microscopy. At least two proteins, 32 kD and 78 kD, can be detected by Western blot analysis of both heart and skeletal muscle, suggesting the existence of alternative forms of the protein. In fact, several forms were found that appear to be the result of alternative splicing. The transcript coding for this Z-band alternatively spliced PDZ motif (ZASP) protein maps on chromosome 10q22.3-10q23.2, near the locus for infantile-onset spinocerebellar ataxia.
Figures




References
-
- Adams M.E., Butler M.H., Dwyer T.M., Peters M.F., Murnane A.A., Froehner S.C. Two forms of mouse syntrophin, a 58-kd dystrophin-associated protein, differ in primary structure and tissue distribution. Neuron. 1993;11:531–540. - PubMed
-
- Agatep, R., R.D. Kirkpatrick, D.L. Parchaliuk, R.A. Woods, and R.D. Gietz. 1998. Transformation of Saccharomyces cerevisiae by the lithium acetate/single-stranded carrier DNA/polyethylene glycol protocol. Technical Tips Online. (http://tto.biomednet.com) 01525.
-
- Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K. Current Protocols in Molecular Biology. Greene Publishing Associates and Wiley-Interscience; New York: 1994.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

