Anti-human immunodeficiency virus type 1 activity, intracellular metabolism, and pharmacokinetic evaluation of 2'-deoxy-3'-oxa-4'-thiocytidine
- PMID: 10428900
- PMCID: PMC89378
- DOI: 10.1128/AAC.43.8.1835
Anti-human immunodeficiency virus type 1 activity, intracellular metabolism, and pharmacokinetic evaluation of 2'-deoxy-3'-oxa-4'-thiocytidine
Abstract
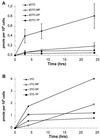
The racemic nucleoside analogue 2'-deoxy-3'-oxa-4'-thiocytidine (dOTC) is in clinical development for the treatment of human immunodeficiency virus (HIV) type 1 (HIV-1) infection. dOTC is structurally related to lamivudine (3TC), but the oxygen and sulfur in the furanosyl ring are transposed. Intracellular metabolism studies showed that dOTC is phosphorylated within cells via the deoxycytidine kinase pathway and that approximately 2 to 5% of dOTC is converted into the racemic triphosphate derivatives, which had measurable half-lives (2 to 3 hours) within cells. Both 5'-triphosphate (TP) derivatives of dOTC were more potent than 3TC-TP at inhibiting HIV-1 reverse transcriptase (RT) in vitro. The K(i) values for dOTC-TP obtained against human DNA polymerases alpha, beta, and gamma were 5,000-, 78-, and 571-fold greater, respectively, than those for HIV RT (28 nM), indicating a good selectivity for the viral enzyme. In culture experiments, dOTC is a potent inhibitor of primary isolates of HIV-1, which were obtained from antiretroviral drug-naive patients as well as from nucleoside therapy-experienced (3TC- and/or zidovudine [AZT]-treated) patients. The mean 50% inhibitory concentration of dOTC for drug-naive isolates was 1.76 microM, rising to only 2.53 and 2.5 microM for viruses resistant to 3TC and viruses resistant to 3TC and AZT, respectively. This minimal change in activity is in contrast to the more dramatic changes observed when 3TC or AZT was evaluated against these same viral isolates. In tissue culture studies, the 50% toxicity levels for dOTC, which were determined by using [(3)H]thymidine uptake as a measure of logarithmic-phase cell proliferation, was greater than 100 microM for all cell lines tested. In addition, after 14 days of continuous culture, at concentrations up to 10 microM, no measurable toxic effect on HepG2 cells or mitochondrial DNA replication within these cells was observed. When administered orally to rats, dOTC was well absorbed, with a bioavailability of approximately 77%, with a high proportion (approximately 16.5% of the levels in serum) found in the cerebrospinal fluid.
Figures
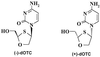

Similar articles
-
Drug resistance and drug combination features of the human immunodeficiency virus inhibitor, BCH-10652 [(+/-)-2'-deoxy-3'-oxa-4'-thiocytidine, dOTC].Antivir Chem Chemother. 2000 Jul;11(4):291-301. doi: 10.1177/095632020001100405. Antivir Chem Chemother. 2000. PMID: 10950391
-
Selection and characterization of HIV-1 variants resistant to the (+) and (-) enantiomers of 2'-deoxy-3'-oxa-4'-thiocytidine (dOTC).Antivir Ther. 1999;4(3):171-7. Antivir Ther. 1999. PMID: 12731757
-
Resistance profiles of (+)2'-deoxy-3'-oxa-4'-thiocytidine and (-)2'-deoxy-3'-oxa-4'-thio-5-fluorocytidine.Nucleosides Nucleotides. 1999 Apr-May;18(4-5):773-8. doi: 10.1080/15257779908041564. Nucleosides Nucleotides. 1999. PMID: 10432679
-
Perspectives on the molecular mechanism of inhibition and toxicity of nucleoside analogs that target HIV-1 reverse transcriptase.Biochim Biophys Acta. 2002 Jul 18;1587(2-3):296-9. doi: 10.1016/s0925-4439(02)00092-3. Biochim Biophys Acta. 2002. PMID: 12084471 Review.
-
Clinical pharmacokinetics of lamivudine.Clin Pharmacokinet. 1999 Jan;36(1):41-66. doi: 10.2165/00003088-199936010-00004. Clin Pharmacokinet. 1999. PMID: 9989342 Review.
Cited by
-
Variations in reverse transcriptase and RNase H domain mutations in human immunodeficiency virus type 1 clinical isolates are associated with divergent phenotypic resistance to zidovudine.Antimicrob Agents Chemother. 2007 Nov;51(11):3861-9. doi: 10.1128/AAC.00646-07. Epub 2007 Aug 27. Antimicrob Agents Chemother. 2007. PMID: 17724152 Free PMC article.
-
Absolute bioavailability and disposition of (-) and (+) 2'-deoxy- 3'-oxa-4'-thiocytidine (dOTC) following single intravenous and oral doses of racemic dOTC in humans.Antimicrob Agents Chemother. 2000 Jun;44(6):1609-15. doi: 10.1128/AAC.44.6.1609-1615.2000. Antimicrob Agents Chemother. 2000. PMID: 10817717 Free PMC article.
-
Safety, tolerability, and pharmacokinetics of single oral doses of BCH-10652 in healthy adult males.Antimicrob Agents Chemother. 2000 Oct;44(10):2816-23. doi: 10.1128/AAC.44.10.2816-2823.2000. Antimicrob Agents Chemother. 2000. PMID: 10991865 Free PMC article. Clinical Trial.
-
Apricitabine does not select additional drug resistance mutations in tissue culture in human immunodeficiency virus type 1 variants containing K65R, M184V, or M184V plus thymidine analogue mutations.Antimicrob Agents Chemother. 2009 Apr;53(4):1683-5. doi: 10.1128/AAC.01168-08. Epub 2009 Feb 17. Antimicrob Agents Chemother. 2009. PMID: 19223637 Free PMC article.
-
Multiple-dose pharmacokinetics of apricitabine, a novel nucleoside reverse transcriptase inhibitor, in patients with HIV-1 infection.Clin Drug Investig. 2008;28(2):129-38. doi: 10.2165/00044011-200828020-00007. Clin Drug Investig. 2008. PMID: 18211121 Clinical Trial.
References
-
- Belleau B, Brasili L, Chan L, DiMarco M, Zacharie B, Nguyen-Ba N. A novel class of 7,3-oxathiolanucleotide analogues having potent anti-HIV activity. Bioorg Med Chem Lett. 1993;3:1723–1728.
-
- Cameron J M, Collis P, Daniel M, Storer R, Wilcox P. Lamivudine. Drugs Future. 1993;18:319–323.
-
- Chang C N, Skalski V, Zhou J H, Cheng Y-C. Biochemical pharmacology of (+) and (−) 2′,3′-dideoxy-3′-thiacytidine as anti-hepatitis B virus agents. J Biol Chem. 1992;267:22414–22420. - PubMed
-
- Daluge S M, Good S S, Faletto M B, Miller W H, St. Clair M H, Boone L R, Tisdale M, Parry N R, Reardon J E, Dornsife R E, Averett D R, Krenitsky T A. 1592U89, a novel carbocyclic nucleoside analog with potent, selective anti-human immunodeficiency virus activity. Antimicrob Agents Chemother. 1997;41:1082–1093. - PMC - PubMed
-
- Furman P A, Fyfe J A, St. Clair M H, Weinhold K, Rideout J L, Freeman G A, Nussinoff Lehrman S, Bolognesi D P, Broder S, Mitsuya H, Barry D W. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci USA. 1986;83:8333–8337. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

