Anergy and cytokine-mediated suppression as distinct superantigen-induced tolerance mechanisms in vivo
- PMID: 10429670
- PMCID: PMC2195559
- DOI: 10.1084/jem.190.1.53
Anergy and cytokine-mediated suppression as distinct superantigen-induced tolerance mechanisms in vivo
Abstract
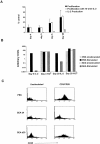
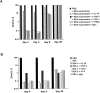
Recombinant-activating gene 2 (RAG-2-/-) T cell receptor-transgenic mice repeatedly injected with the superantigen staphylococcal enterotoxin A entered a tolerant state in which splenic CD4+ T cells produced little interleukin (IL)-2, interferon gamma, or IL-4. This state resulted from a combination of both clonal anergy and cytokine-mediated immunosuppression. The anergy persisted for at least 3 wk and could be distinguished from the suppression by a decrease in IL-2 production per cell, a block in the activation of early response kinases, and a failure to be reversed with anti-transforming growth factor (TGF)-beta. Full suppression lasted for only 1 wk and involved both IL-10 and TGF-beta, but required additional unknown molecules for optimal effect. These experiments show that complex in vivo interactions of multiple peripheral tolerance mechanisms can now be dissected at both the cellular and molecular levels.
Figures
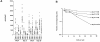
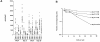



Similar articles
-
Effect of treatments with cyclosporin A and anti-interferon-gamma antibodies on the mechanisms of immune tolerance in staphylococcal enterotoxin B primed mice.Scand J Immunol. 1997 Nov;46(5):459-68. doi: 10.1046/j.1365-3083.1997.d01-152.x. Scand J Immunol. 1997. PMID: 9393628
-
Unique cytokine production profile of anergic human T cells in SCID-hu mice after staphylococcal enterotoxin B administration.J Immunol. 1995 Apr 1;154(7):3204-12. J Immunol. 1995. PMID: 7534791
-
Superantigens anergize cytokine production but not cytotoxicity in vivo.Immunology. 1994 May;82(1):117-25. Immunology. 1994. PMID: 7913912 Free PMC article.
-
Immunoregulatory mechanisms of T-cell-dependent shock induced by a bacterial superantigen in mice.Infect Immun. 1996 Sep;64(9):3443-5. doi: 10.1128/iai.64.9.3443-3445.1996. Infect Immun. 1996. PMID: 8751882 Free PMC article. Review. No abstract available.
-
Superantigen-induced regulatory T cells in vivo.Chem Immunol Allergy. 2007;93:137-160. doi: 10.1159/000100862. Chem Immunol Allergy. 2007. PMID: 17369704 Review.
Cited by
-
Naive T cells proliferate strongly in neonatal mice in response to self-peptide/self-MHC complexes.Proc Natl Acad Sci U S A. 2002 Apr 2;99(7):4538-43. doi: 10.1073/pnas.062621699. Epub 2002 Mar 26. Proc Natl Acad Sci U S A. 2002. PMID: 11917110 Free PMC article.
-
Staphylococcal enterotoxins in the etiopathogenesis of mucosal autoimmunity within the gastrointestinal tract.Toxins (Basel). 2014 Apr 25;6(5):1471-89. doi: 10.3390/toxins6051471. Toxins (Basel). 2014. PMID: 24776983 Free PMC article. Review.
-
Long-term staphylococcal enterotoxin C1 exposure induces soluble factor-mediated immunosuppression by bovine CD4+ and CD8+ T cells.Infect Immun. 2007 Jan;75(1):260-9. doi: 10.1128/IAI.01358-06. Epub 2006 Oct 9. Infect Immun. 2007. PMID: 17030573 Free PMC article.
-
Temporal sequence and kinetics of proinflammatory and anti-inflammatory cytokine secretion induced by toxic shock syndrome toxin 1 in human peripheral blood mononuclear cells.Infect Immun. 2001 Dec;69(12):7544-9. doi: 10.1128/IAI.69.12.7544-7549.2001. Infect Immun. 2001. PMID: 11705931 Free PMC article.
-
Anergy and suppression as coexistent mechanisms for the maintenance of peripheral T cell tolerance.Immunol Res. 2003;27(2-3):295-302. doi: 10.1385/IR:27:2-3:295. Immunol Res. 2003. PMID: 12857976 Review.
References
-
- Ramsdell F., Fowlkes B.J. Clonal deletion versus clonal anergythe role of the thymus in inducing self tolerance. Science. 1990;248:1342–1348. - PubMed
-
- Moskophidis D., Lechner F., Pircher H., Zinkernagel R.M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. - PubMed
-
- Rammensee H.G., Kroschewski R., Frangoulis B. Clonal anergy induced in mature V beta 6+ T lymphocytes on immunizing Mls-1 b mice with Mls-1 a expressing cells. Nature. 1989;339:541–544. - PubMed
-
- Webb S.R., Hutchinson J., Hayden K., Sprent J. Expansion/deletion of mature T cells exposed to endogenous superantigen in vivo. J. Immunol. 1994;152:586–596. - PubMed
-
- McCormack J.E., Callahan J.E., Kappler J., Marrack P.C. Profound deletion of mature T cells in vivo by chronic exposure to endogenous superantigen. J. Immunol. 1993;150:3785–3792. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

