Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response
- PMID: 10429676
- PMCID: PMC2195555
- DOI: 10.1084/jem.190.1.125
Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response
Abstract
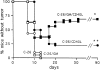
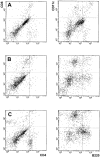
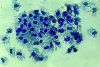
We transduced BALB/c-derived C-26 colon carcinoma cells with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand (CD40L) genes to favor interaction of these cells with host dendritic cells (DCs) and, therefore, cross-priming. Cotransduced cells showed reduced tumorigenicity, and tumor take was followed by regression in some mice. In vivo tumors were heavily infiltrated with DCs that were isolated, phenotyped, and tested in vitro for stimulation of tumor-specific cytotoxic T lymphocytes (CTLs). BALB/c C-26 carcinoma cells express the endogenous murine leukemia virus (MuLV) env gene as a tumor-associated antigen. This antigen is shared among solid tumors of BALB/c and C57BL/6 mice and contains two epitopes, AH-1 and KSP, recognized in the context of major histocompatibility complex class I molecules H-2Ld and H-2K(b), respectively. DCs isolated from C-26/GM/CD40L tumors grown in (BALB/c x C57BL/6)F1 mice (H-2d x b) stimulated interferon gamma production by both anti-AH-1 and KSP CTLs, whereas tumor-infiltrating DCs (TIDCs) of BALB/c mice stimulated only anti-AH-1 CTLs. Furthermore, TIDCs primed naive mice for CTL activity as early as 2 d after injection into the footpad, whereas double-transduced tumor cells required at least 5 d for priming; this difference may reflect direct DC priming versus indirect tumor cell priming. Immunohistochemical staining indicated colocalization of DCs and apoptotic bodies in the tumors. These data indicate that DCs infiltrating tumors that produce GM-CSF and CD40L can capture cellular antigens, likely through uptake of apoptotic bodies, and mature in situ to a stage suitable for antigen presentation. Thus, tumor cell-based vaccines engineered to favor the interaction with host DCs can be considered.
Figures



References
-
- Steinman R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. - PubMed
-
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Thurnher M., Radmayr C., Ramoner R., Ebner S., Bock G., Klocker H., Romani N., Bartsch G. Human renal-cell carcinoma tissue contains dendritic cells. Int. J. Cancer. 1996;67:1–7. - PubMed
-
- Becker Y. Anticancer role of dendritic cells (DC) in human and experimental cancers—a review. Anticancer Res. 1992;12:511–520. - PubMed
-
- Huang A.Y., Golumbek P., Ahmadzadeh M., Jaffee E., Pardoll D., Levitsky H. Role of bone marrow-derived cells in presenting MHC-I-restricted tumor antigens. Science. 1994;264:961–965. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

