Hypoxia-reoxygenation triggers coronary vasospasm in isolated bovine coronary arteries via tyrosine nitration of prostacyclin synthase
- PMID: 10429677
- PMCID: PMC2195551
- DOI: 10.1084/jem.190.1.135
Hypoxia-reoxygenation triggers coronary vasospasm in isolated bovine coronary arteries via tyrosine nitration of prostacyclin synthase
Abstract
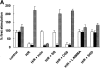
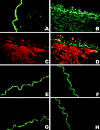
The role of peroxynitrite in hypoxia-reoxygenation-induced coronary vasospasm was investigated in isolated bovine coronary arteries. Hypoxia-reoxygenation selectively blunted prostacyclin (PGI2)-dependent vasorelaxation and elicited a sustained vasoconstriction that was blocked by a cyclooxygenase inhibitor, indomethacin, and SQ29548, a thromboxane (Tx)A2/prostaglandin H2 receptor antagonist, but not by CGS13080, a TxA2 synthase blocker. The inactivation of PGI2 synthase, as evidenced by suppressed 6-keto-PGF1 alpha release and a decreased conversion of 14C-prostaglandin H2 into 6-keto-PGF1 alpha, was paralleled by an increased nitration in both vascular endothelium and smooth muscle of hypoxia-reoxygenation-exposed vessels. The administration of the nitric oxide (NO) synthase inhibitors as well as polyethylene-glycolated superoxide dismutase abolished the vasospasm by preventing the inactivation and nitration of PGI2 synthase, suggesting that peroxynitrite was implicated. Moreover, concomitant administration to the organ baths of the two precursors of peroxynitrite, superoxide, and NO mimicked the effects of hypoxia-reoxygenation, although none of them were effective when given separately. We conclude that hypoxia-reoxygenation elicits the formation of superoxide, which causes loss of the vasodilatory action of NO and at the same time yields peroxynitrite. Subsequently, peroxynitrite nitrates and inactivates PGI2 synthase, leaving unmetabolized prostaglandin H2, which causes vasospasm, platelet aggregation, and thrombus formation via the TxA2/prostaglandin H2 receptor.
Figures




References
-
- Vane J.R., Anggard E.E., Botting R.M. Regulatory function of the vascular endothelium. N. Engl. J. Med. 1990;323:27–36. - PubMed
-
- Moncada S., Palmer R.M., Higgs E.A. Nitric oxidephysiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. - PubMed
-
- Moncada S., Vane J.R. Pharmacology and endogenous roles of endoperoxide prostaglandins, thromboxane A2 and prostacyclin. N. Engl. J. Med. 1979;300:1142–1147.
-
- Zou M.H., Ullrich V. Peroxynitrite formed by simultaneous generation of nitric oxide and superoxide selectively inhibits bovine aortic prostacyclin synthase. FEBS Lett. 1996;382:101–104. - PubMed

