Mixed chimerism induced without lethal conditioning prevents T cell- and anti-Gal alpha 1,3Gal-mediated graft rejection
- PMID: 10430609
- PMCID: PMC408419
- DOI: 10.1172/JCI6656
Mixed chimerism induced without lethal conditioning prevents T cell- and anti-Gal alpha 1,3Gal-mediated graft rejection
Abstract
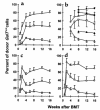
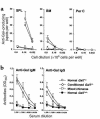
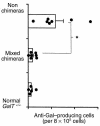
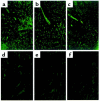
Gal alpha 1,3Gal-reactive (Gal-reactive) antibodies are a major impediment to pig-to-human xenotransplantation. We investigated the potential to induce tolerance of anti-Gal-producing cells and prevent rejection of vascularized grafts in the combination of alpha 1,3-galactosyltransferase wild-type (GalT(+/+)) and deficient (GalT(-/-)) mice. Allogeneic (H-2 mismatched) GalT(+/+) bone marrow transplantation (BMT) to GalT(-/-) mice conditioned with a nonmyeloablative regimen, consisting of depleting CD4 and CD8 mAb's and 3 Gy whole-body irradiation and 7 Gy thymic irradiation, led to lasting multilineage H-2(bxd) GalT(+/+) + H-2(d) GalT(-/-) mixed chimerism. Induction of mixed chimerism was associated with a rapid reduction of serum anti-Gal naturally occurring antibody levels. Anti-Gal-producing cells were undetectable by 2 weeks after BMT, suggesting that anti-Gal-producing cells preexisting at the time of BMT are rapidly tolerized. Even after immunization with Gal-bearing xenogeneic cells, mixed chimeras were devoid of anti-Gal-producing cells and permanently accepted donor-type GalT(+/+) heart grafts (>150 days), whereas non-BMT control animals rejected these hearts within 1-7 days. B cells bearing receptors for Gal were completely absent from the spleens of mixed chimeras, suggesting that clonal deletion and/or receptor editing may maintain B-cell tolerance to Gal. These findings demonstrate the principle that induction of mixed hematopoietic chimerism with a potentially relevant nonmyeloablative regimen can simultaneously lead to tolerance among both T cells and Gal-reactive B cells, thus preventing vascularized xenograft rejection.
Figures





Comment in
-
Tolerance by transplantation: how much is enough, how much is too much?J Clin Invest. 1999 Aug;104(3):227-8. doi: 10.1172/JCI7850. J Clin Invest. 1999. PMID: 10430603 Free PMC article. Review. No abstract available.
References
-
- Platt JL. Xenotransplantation: recent progress and current perspectives. Curr Opin Immunol. 1996;8:721–728. - PubMed
-
- Sachs DH. The pig as a xenograft donor. Pathol Biol. 1994;42:217–219. - PubMed
-
- Oriol R, Ye Y, Koren E, Cooper DKC. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation. Transplantation. 1993;56:1433–1442. - PubMed
-
- Galili U. Interaction of the natural anti-Gal antibody with α-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today. 1993;14:480–482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

