Role of the tyrosine kinase pyk2 in the integrin-dependent activation of human neutrophils by TNF
- PMID: 10430614
- PMCID: PMC408415
- DOI: 10.1172/JCI6018
Role of the tyrosine kinase pyk2 in the integrin-dependent activation of human neutrophils by TNF
Abstract
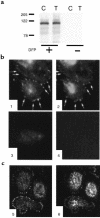
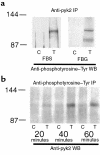
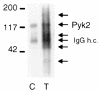
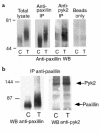
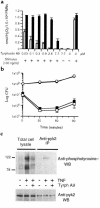
Secretion of inflammatory products from neutrophils can be induced by a combination of signals from ligated integrins and receptors for soluble, physiological agonists such as TNF. Here we identify pyk2 in primary human neutrophils; localize it to focal adhesions and podosomes; and demonstrate its tyrosine phosphorylation, activation, and association with paxillin during stimulation of adherent cells by TNF. Tyrphostin A9 emerged as the most potent and selective of 51 tyrosine kinase inhibitors tested against the TNF-induced respiratory burst. Tyrphostin A9 inhibited TNF-induced tyrosine phosphorylation of pyk2 without blocking the cells' bactericidal activity. Wortmannin, an inhibitor of phosphatidylinositol-3-kinase, potently blocked the TNF-induced respiratory burst and selectively inhibited tyrosine phosphorylation of pyk2. Thus, pyk2 appears to play an essential role in the ability of neutrophils to integrate signals from beta(2) integrins and TNF receptors.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

