The Gly972-->Arg amino acid polymorphism in IRS-1 impairs insulin secretion in pancreatic beta cells
- PMID: 10430617
- PMCID: PMC408413
- DOI: 10.1172/JCI5870
The Gly972-->Arg amino acid polymorphism in IRS-1 impairs insulin secretion in pancreatic beta cells
Abstract
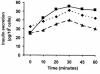
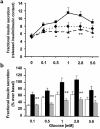
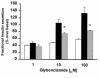
Recent studies have identified several polymorphisms in the human insulin receptor substrate-1 (IRS-1) gene. The most prevalent IRS-1 variant, a Gly-->Arg change at the codon 972, has been reported to be increased in prevalence among patients with type 2 diabetes. Carriers of the Arg(972) substitution are characterized by lower fasting insulin and C-peptide levels compared with non-carriers, suggesting that the Arg(972) IRS-1 variant may contribute to impairment of insulin secretion. In this study, we stably overexpressed both wild-type IRS-1 (RIN-WT) and Arg(972) IRS-1 variant (RIN-Arg(972)) in RIN beta cells to investigate directly whether the polymorphism in codon 972 of IRS-1 impairs insulin secretion. The Arg(972) IRS-1 variant did not affect expression or function of endogenous IRS-2. RIN-WT showed a marked increase in both glucose- and insulin-stimulated tyrosine phosphorylation of IRS-1 compared with control RIN cells. The Arg(972) IRS-1 variant did not alter the extent of either glucose- or insulin-stimulated tyrosine phosphorylation of recombinant IRS-1. However, RIN-Arg(972) showed a significant decrease in binding of the p85 subunit of phosphatidylinositol-3-kinase (PI 3-kinase) with IRS-1, compared with RIN-WT. Compared with control RIN cells, insulin content was reduced to the same extent in RIN-WT or RIN-Arg(972) at both the protein and mRNA levels. Both glucose- and sulfonylurea-induced insulin secretion was increased in RIN-WT compared with control RIN cells. By contrast, RIN cells expressing Arg(972) IRS-1 exhibited a marked decrease in both glucose- and sulfonylurea-stimulated insulin secretion compared with RIN-WT. These data suggest that the insulin signaling pathway involving the IRS-1/PI 3-kinase may play an important role in the insulin secretory process in pancreatic beta cells. More importantly, the results suggest that the common Arg(972) IRS-1 polymorphism may impair glucose-stimulated insulin secretion, thus contributing to the relative insulin deficiency observed in carriers of this variant.
Figures




References
-
- De Fronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Reviews. 1997;5:177–269.
-
- Newmann B, et al. Concordance for type 2 (non-insulin-dependent) diabetes mellitus in male twins. Diabetologia. 1987;30:763–768. - PubMed
-
- Lillioja S, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. - PubMed
-
- Rich SS. Mapping genes in diabetes: genetic epidemiological perspective. Diabetes. 1990;39:1315–1319. - PubMed
-
- Martin BC, et al. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340:925–929. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

