A role for dipeptidyl peptidase IV in suppressing the malignant phenotype of melanocytic cells
- PMID: 10430620
- PMCID: PMC2195594
- DOI: 10.1084/jem.190.3.311
A role for dipeptidyl peptidase IV in suppressing the malignant phenotype of melanocytic cells
Abstract
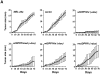
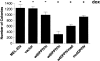
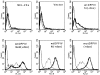
Dipeptidyl peptidase IV (DPPIV) is a cell surface peptidase expressed by normal melanocytes, epithelial cells, and other cells. Malignant cells, including melanomas and carcinomas, frequently lose or alter DPPIV cell surface expression. Loss of DPPIV expression occurs during melanoma progression at a stage where transformed melanocytes become independent of exogenous growth factors for survival. Tetracycline-inducible expression vectors were constructed to express DPPIV in human melanoma cells. Reexpressing DPPIV in melanoma cells at or below levels expressed by normal melanocytes induced a profound change in phenotype that was characteristic of normal melanocytes. DPPIV expression led to a loss of tumorigenicity, anchorage-independent growth, a reversal in a block in differentiation, and an acquired dependence on exogenous growth factors for cell survival. Suppression of tumorigenicity and reversal of a block in differentiation were dependent on serine protease activity, assessed using mutant DPPIV molecules containing serine-->alanine substitutions. Surprisingly, dependence on exogenous growth factors was not dependent on serine protease activity. Reexpression of either wild-type or mutant DPPIV rescued expression of a second putative cell surface serine peptidase, fibroblast activation protein alpha, which can form a heterodimer with DPPIV. This observation suggests that rescue of fibroblast activation protein alpha may play a role in regulating growth of melanocytic cells. These results support the view that downregulation of DPPIV is an important early event in the pathogenesis of melanoma.
Figures









Comment in
-
CD26/dipeptidyl peptidase IV in context. The different roles of a multifunctional ectoenzyme in malignant transformation.J Exp Med. 1999 Aug 2;190(3):301-6. doi: 10.1084/jem.190.3.301. J Exp Med. 1999. PMID: 10430618 Free PMC article. Review. No abstract available.
References
-
- Schrader W.P., Stacy A.R. Purification and subunit structure of adenosine deaminase from human kidney. J. Biol. Chem. 1977;252:6409–6415. - PubMed
-
- Morimoto C., Schlossman S. The structure and function of CD26 in the T-cell immune response. Immunol. Rev. 1998;61:55–70. - PubMed
-
- Schrader W.P., West C.A., Miczek A.D., Norton E.K. Characterization of the adenosine deaminase-adenosine deaminase complexing protein binding reaction. J. Biol. Chem. 1990;265:19312–19318. - PubMed
-
- Schrader W.P., Pollara B. Localization of an adenosine deaminase-binding protein in human kidney. J. Lab. Clin. Med. 1978;92:656–662. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

