Focal adhesion kinase promotes phospholipase C-gamma1 activity
- PMID: 10430888
- PMCID: PMC17725
- DOI: 10.1073/pnas.96.16.9021
Focal adhesion kinase promotes phospholipase C-gamma1 activity
Abstract
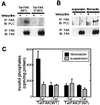
The nonreceptor tyrosine kinase FAK ("focal adhesion kinase") is a key mediator of integrin signaling events controlling cellular responses to the extracellular matrix, including spreading, migration, proliferation, and survival. Integrin-ligand interactions stimulate FAK tyrosine phosphorylation and activation of FAK signaling functions. Here evidence is presented that the FAK autophosphorylation site Tyr-397 mediates a direct interaction with the C-terminal Src homology 2 domain of phospholipase C (PLC)-gamma1 and that this is required for both adhesion-dependent association of the two molecules and increased inositol phosphate production in mouse embryo fibroblasts. Overexpression of FAK and PLC-gamma1 in COS-7 cells increases PLC-gamma1 enzymatic activity and tyrosine phosphorylation, also dependent on FAK Tyr-397. However, FAK appears incapable of directly phosphorylating PLC-gamma1. These observations suggest a role for FAK in recruiting PLC-gamma1 to the plasma membrane at sites of cell-matrix adhesion and there promoting its enzymatic activity, possibly by releasing the repression caused by intramolecular interactions of the PLC-gamma1 Src homology domains and/or by positioning it for phosphorylation by associated Src-family kinases. These findings expand the known signaling functions of FAK and provide mechanistic insight into integrin-stimulation of PLC-gamma1.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

