The oxyhemoglobin reaction of nitric oxide
- PMID: 10430889
- PMCID: PMC17726
- DOI: 10.1073/pnas.96.16.9027
The oxyhemoglobin reaction of nitric oxide
Abstract
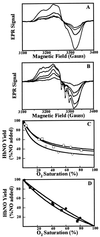
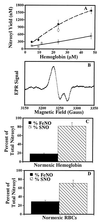
The oxidation of nitric oxide (NO) to nitrate by oxyhemoglobin is a fundamental reaction that shapes our understanding of NO biology. This reaction is considered to be the major pathway for NO elimination from the body; it is the basis for a prevalent NO assay; it is a critical feature in the modeling of NO diffusion in the circulatory system; and it informs a variety of therapeutic applications, including NO-inhalation therapy and blood substitute design. Here we show that, under physiological conditions, this reaction is of little significance. Instead, NO preferentially binds to the minor population of the hemoglobin's vacant hemes in a cooperative manner, nitrosylates hemoglobin thiols, or reacts with liberated superoxide in solution. In the red blood cell, superoxide dismutase eliminates superoxide, increasing the yield of S-nitrosohemoglobin and nitrosylated hemes. Hemoglobin thus serves to regulate the chemistry of NO and maintain it in a bioactive state. These results represent a reversal of the conventional view of hemoglobin in NO biology and motivate a reconsideration of fundamental issues in NO biochemistry and therapy.
Figures


Comment in
-
Physiological reactions of nitric oxide and hemoglobin: a radical rethink.Proc Natl Acad Sci U S A. 1999 Aug 31;96(18):9967-9. doi: 10.1073/pnas.96.18.9967. Proc Natl Acad Sci U S A. 1999. PMID: 10468537 Free PMC article. Review. No abstract available.
References
-
- Kelm M, Yoshida K. In: Metabolic Fate of Nitric Oxide and Related N-Oxides. 1st Ed. Feelisch M, Stamler J S, editors. London: Wiley; 1996. pp. 47–58.
-
- Pietraforte D, Mallozzi C, Scorza G, Minetti M. Biochemistry. 1995;34:7177–7185. - PubMed
-
- Feelisch M, Kubitzek D, Werringloer J. In: The Oxyhemoglobin Assay. 1st Ed. Feelisch M, Stamler J S, editors. London: Wiley; 1996. pp. 455–478.
-
- Traylor T G, Sharma V S. Biochemistry. 1992;31:2847–2849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

