Differential assembly of Cdc45p and DNA polymerases at early and late origins of DNA replication
- PMID: 10430907
- PMCID: PMC17744
- DOI: 10.1073/pnas.96.16.9130
Differential assembly of Cdc45p and DNA polymerases at early and late origins of DNA replication
Abstract
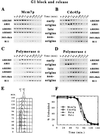
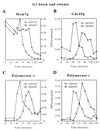
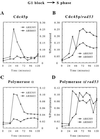
Chromosomes are replicated in characteristic, temporal patterns during S phase. We have compared the timing of association of replication proteins at early- and late-replicating origins of replication. Minichromosome maintenance proteins assemble simultaneously at early- and late-replicating origins. In contrast, Cdc45p association with late origins is delayed relative to early origins. DNA polymerase alpha association is similarly delayed at late origins and requires Cdc45p function. Activation of the S phase checkpoint inhibits association of Cdc45p with late-firing origins. These studies suggest that Cdc45p is poised to serve as a key regulatory target for both the temporal and checkpoint-mediated regulation of replication origins.
Figures


References
-
- Jacob F, Brenner S, Cuzin F. Cold Spring Harbor Symp Quant Biol. 1963;28:329–348.
-
- DePamphilis M L. In: DNA Replication in Eukaryotic Cells. DePamphilis M L, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1996. pp. 45–86.
-
- Diffley J F X. Genes Dev. 1996;10:2819–2830. - PubMed
-
- Diller J D, Raghuraman M K. Trends Biochem Sci. 1994;19:320–325. - PubMed
-
- Simon I, Cedar H. In: DNA Replication in Eukaryotic Cells. DePamphilis M L, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1996. pp. 387–408.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

